Health and Social Care Information Centre
NHS Data Model and Dictionary Service
| Type: | Patch |
| Reference: | 1408 |
| Version No: | 1.0 |
| Subject: | November 2013 Release Patch |
| Effective Date: | Immediate |
| Reason for Change: | Patch |
| Publication Date: | 15 November 2013 |
Background:
This patch updates the NHS Data Model and Dictionary in preparation for the November 2013 Release and includes:
- What's New amended to include Change Requests incorporated since the last version of the NHS Data Model and Dictionary was published
- Missing hyperlinks added
- Website links updated
- Html format corrected.
To view a demonstration on "How to Read an NHS Data Model and Dictionary Change Request", visit the NHS Data Model and Dictionary help pages at: http://www.datadictionary.nhs.uk/Flash_Files/changerequest.htm.
Note: if the web page does not open, please copy the link and paste into the web browser.
Summary of changes:
| Date: | 15 November 2013 |
| Sponsor: | Richard Kavanagh, Head of Data Standards - Interoperability Specifications, Health and Social Care Information Centre |
Note: New text is shown with a blue background. Deleted text is crossed out. Retired text is shown in grey. Within the Diagrams deleted classes and relationships are red, changed items are blue and new items are green.
Click here for a printer friendly view of this page.
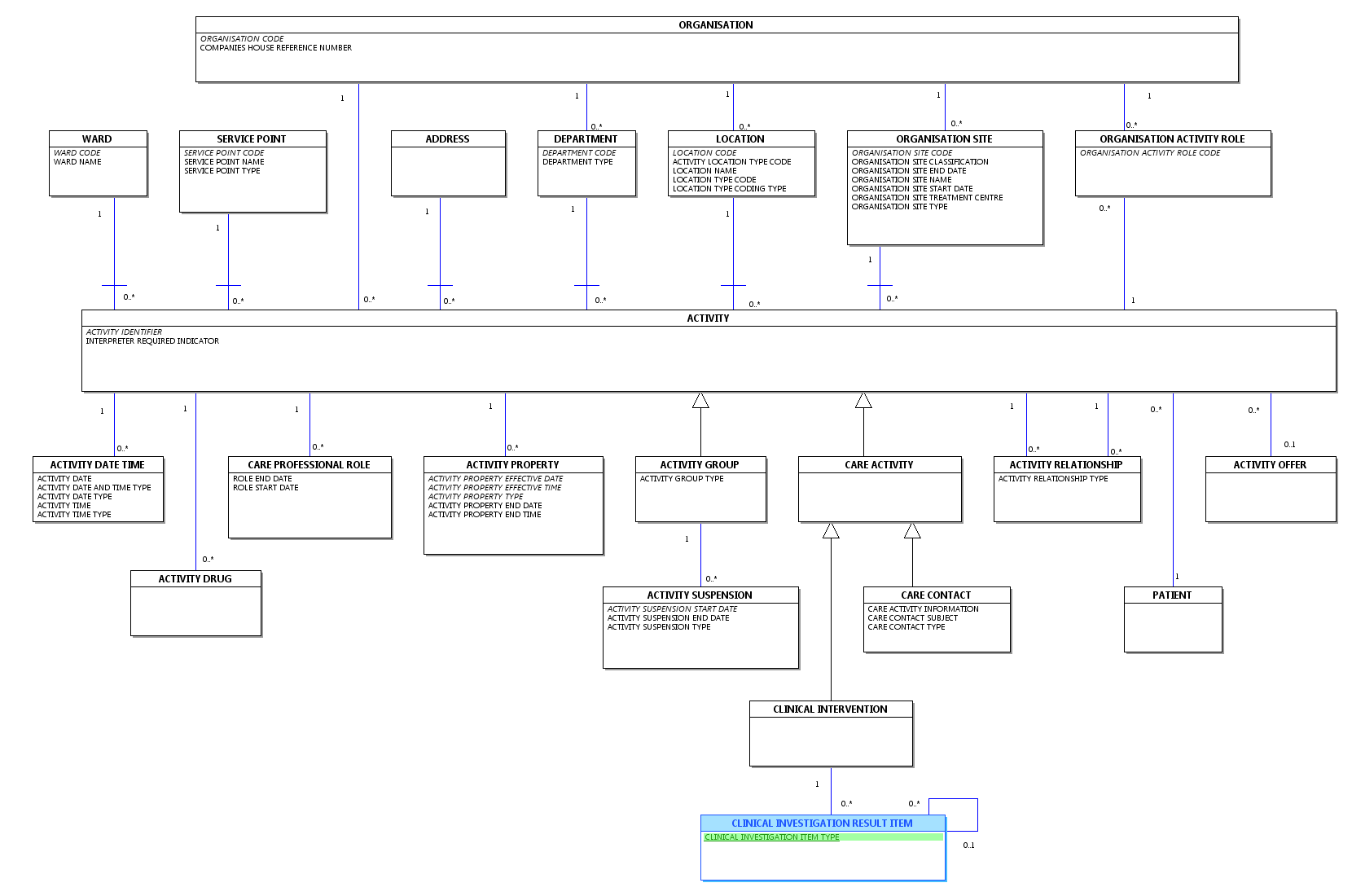
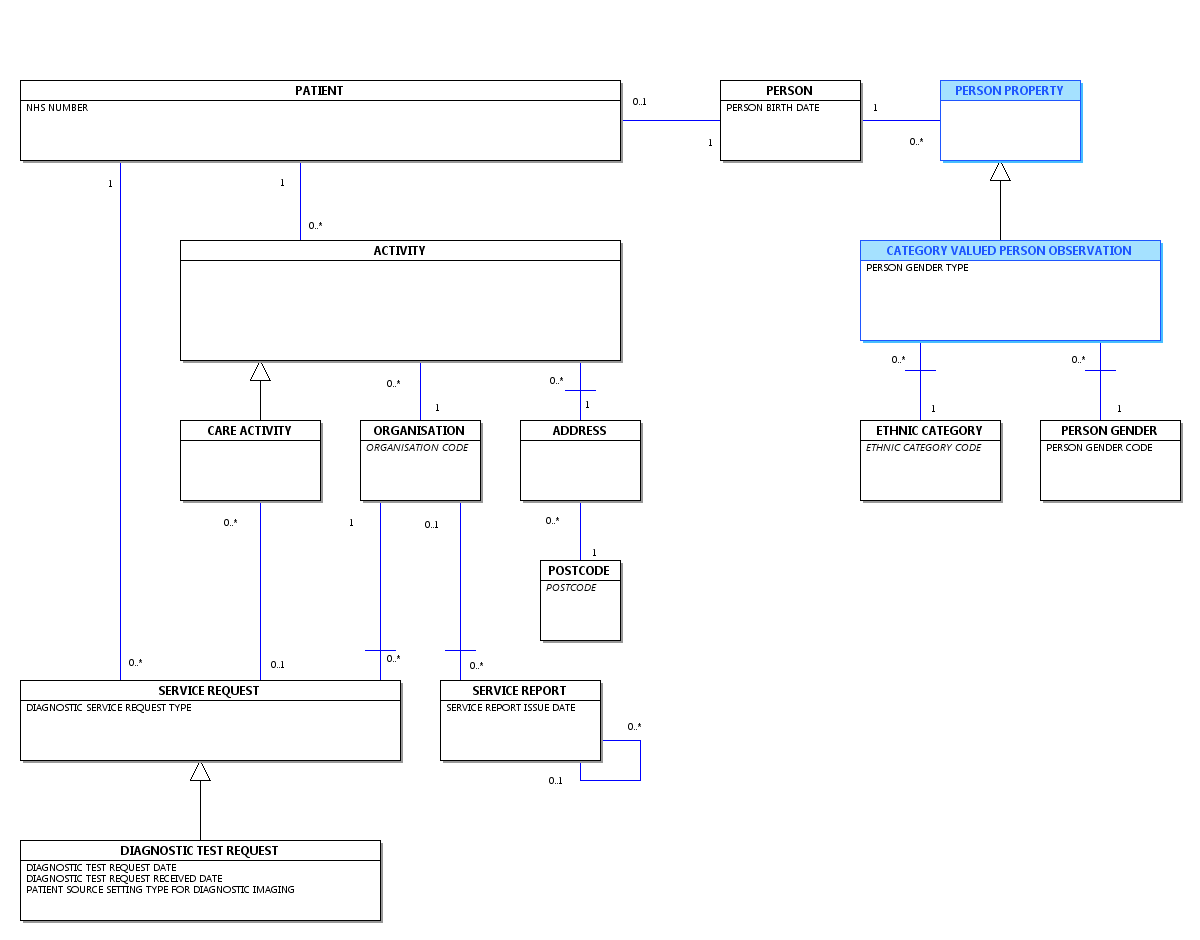
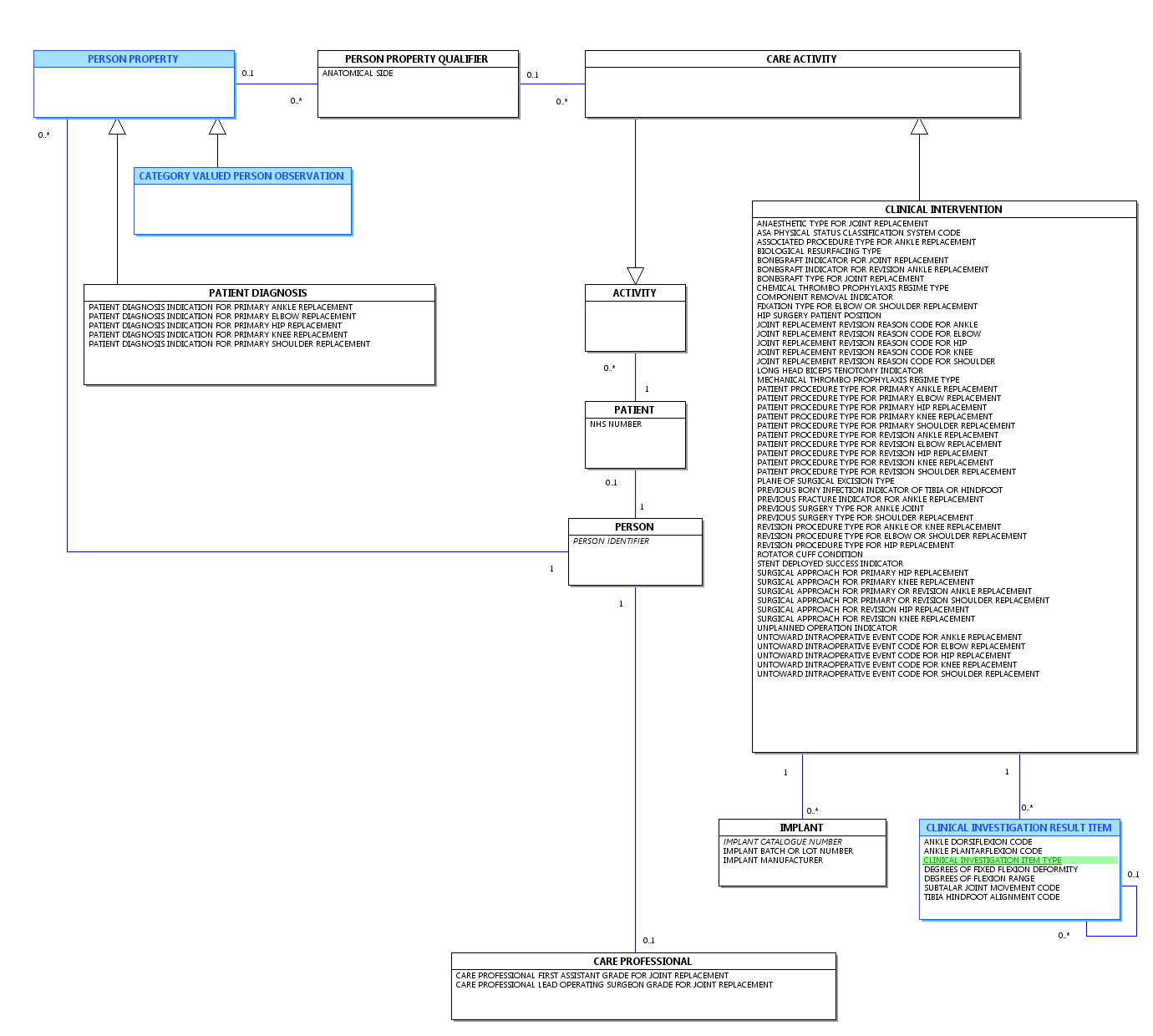
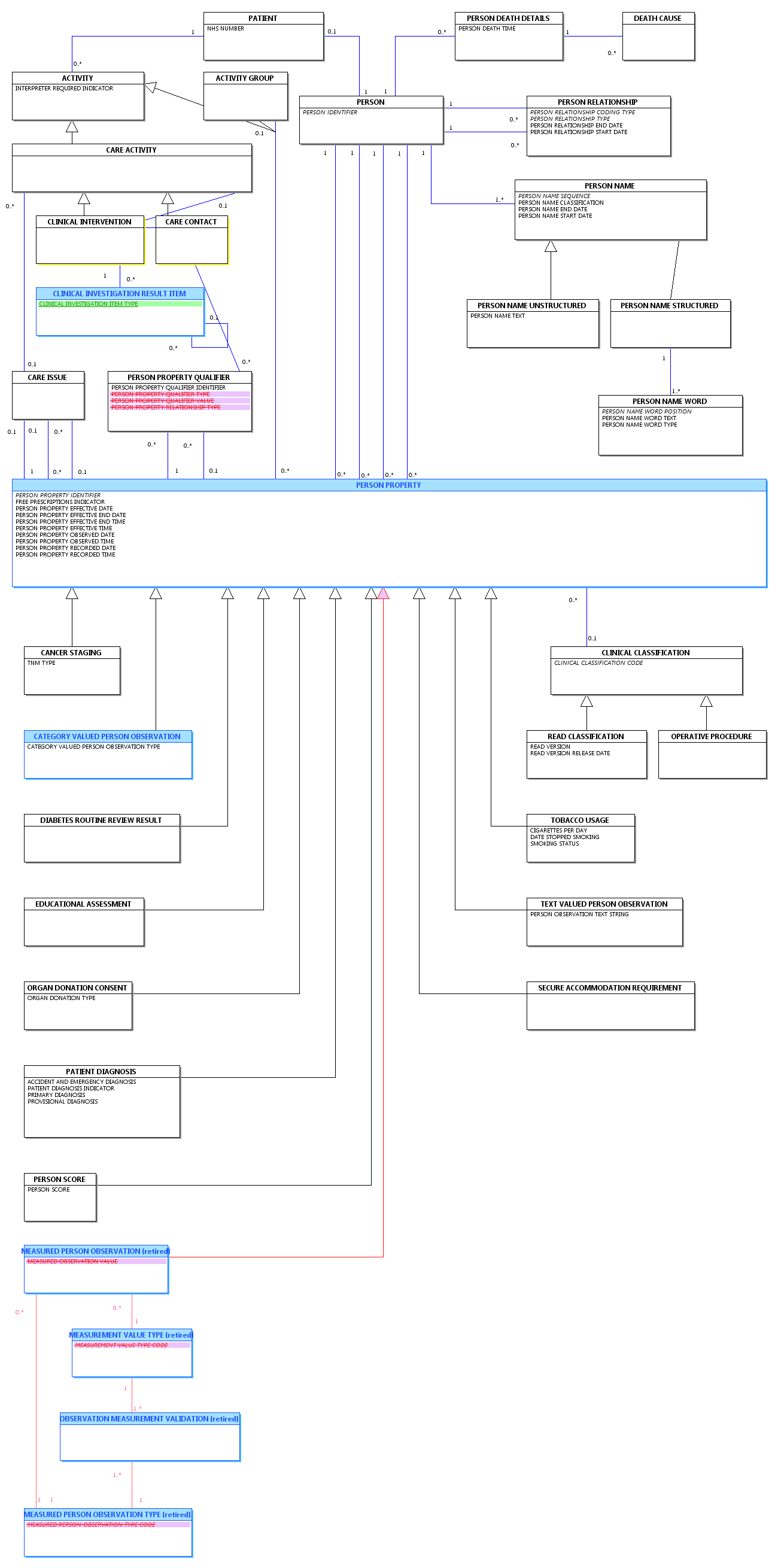
Change to Diagram: Changed Diagram
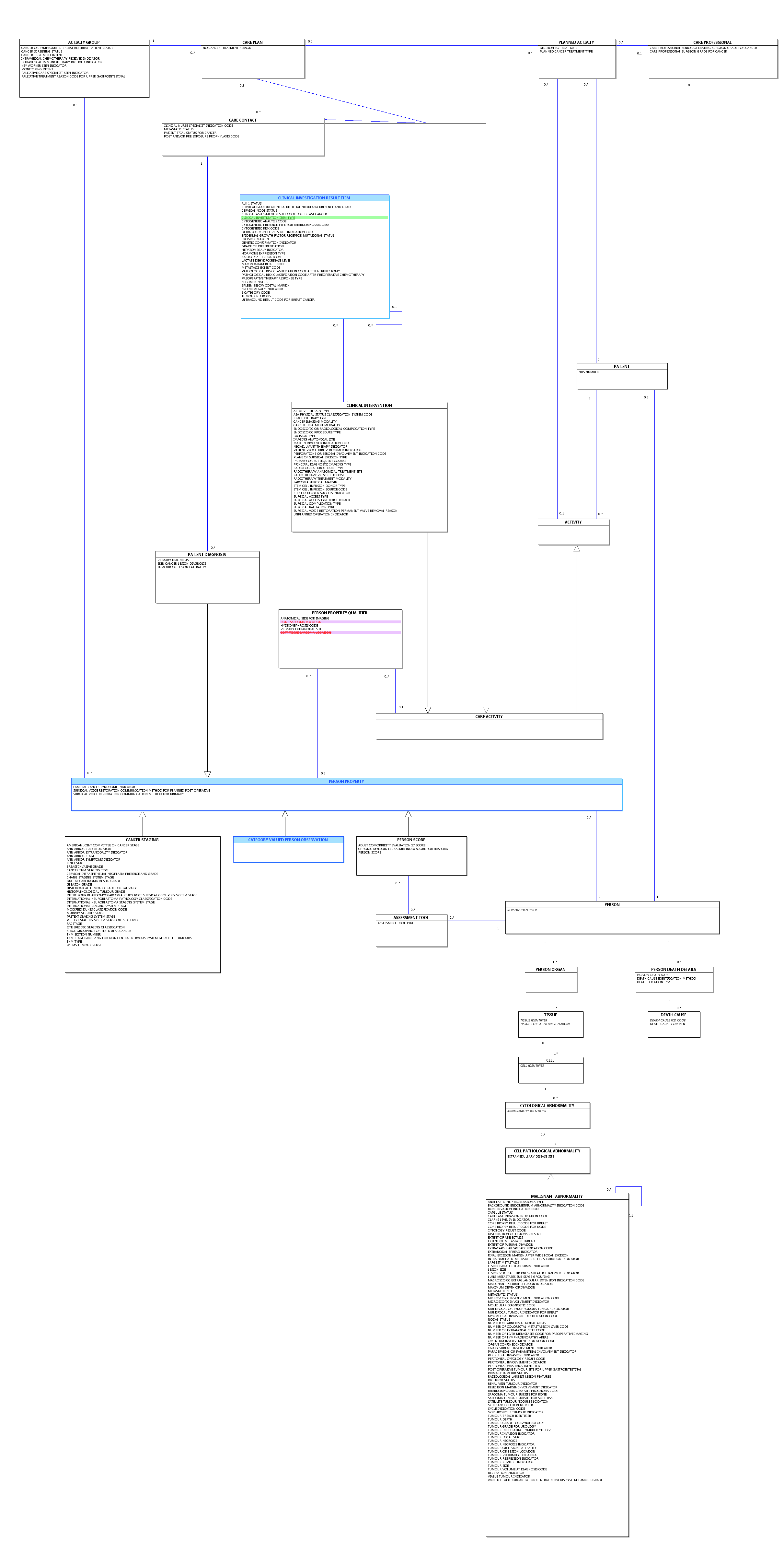
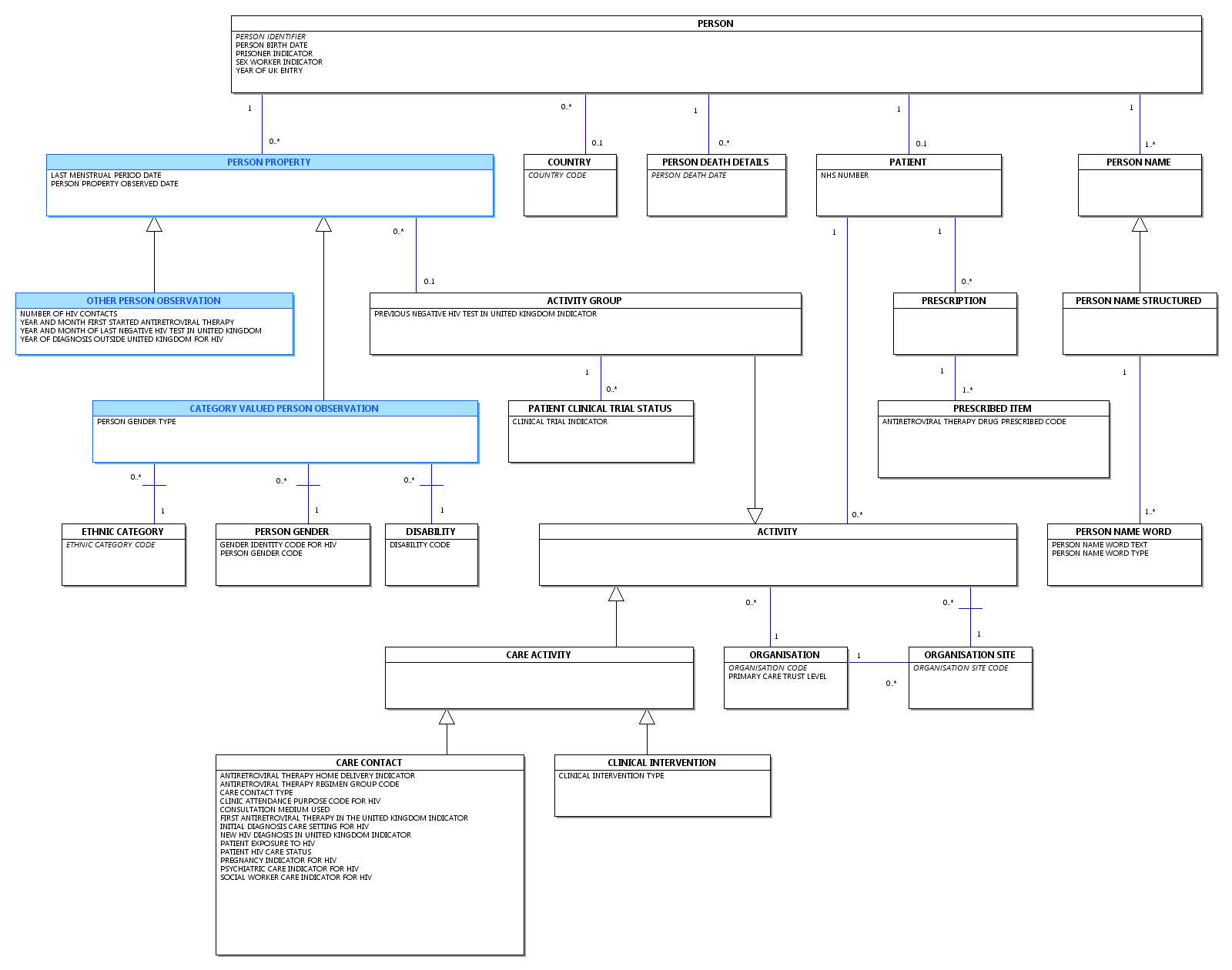
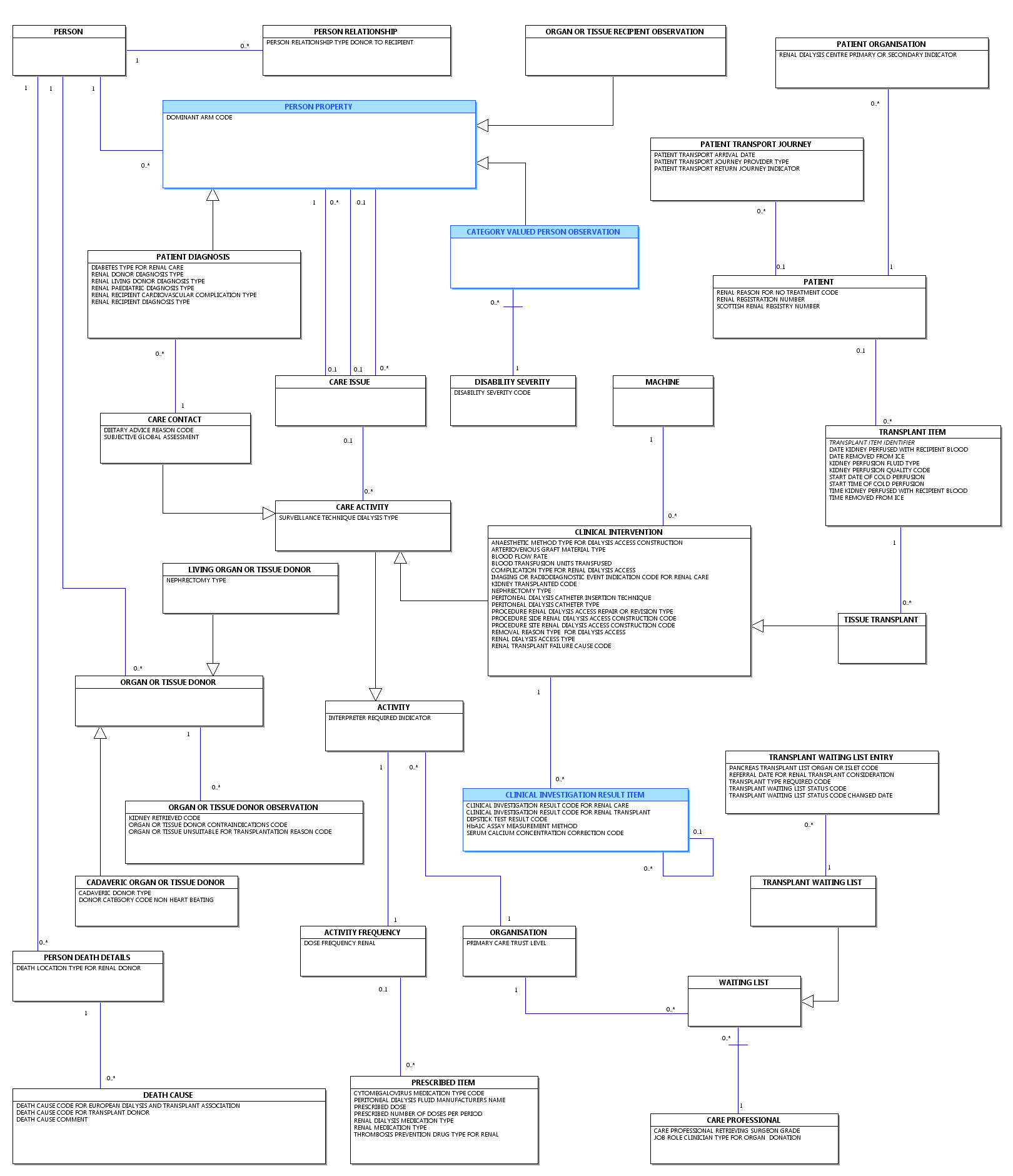
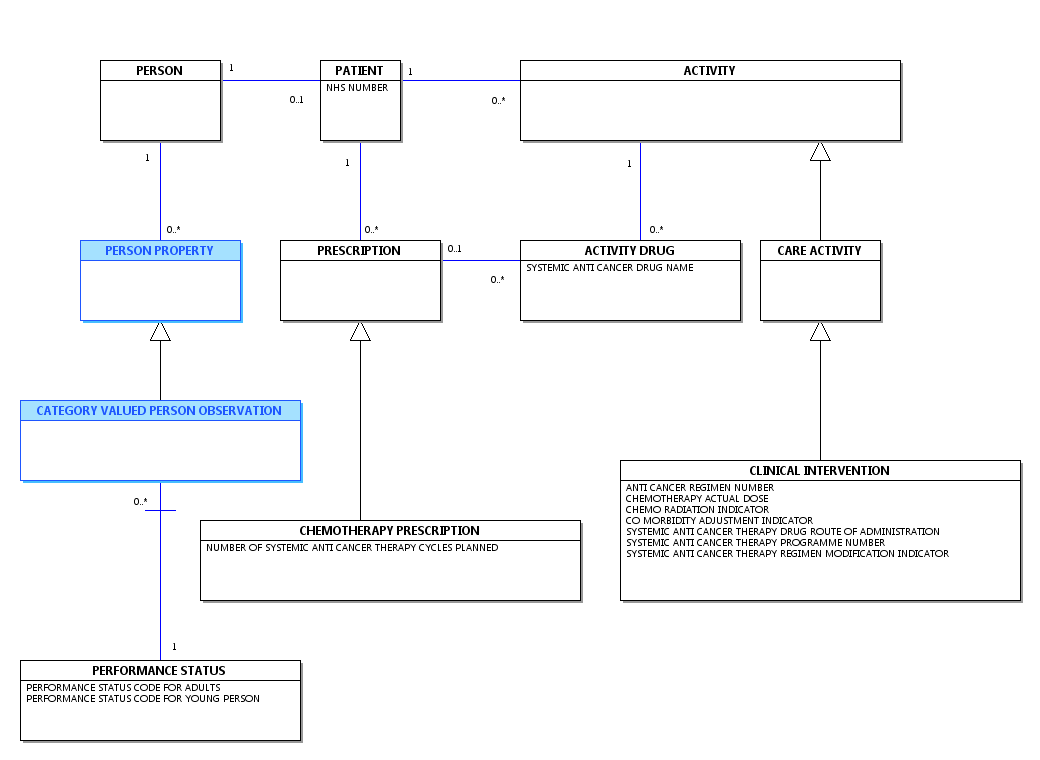
Change to Diagram: Changed Diagram
Change to Data Set: Changed Description
Cancer Outcomes and Services Data Set Overview
The Mandatory, Required, Optional or Not included in the COSDS Message (M/R/O/X) column indicates the recommendation for the inclusion of data.
- M = Mandatory: this data element is mandatory and the technical process (e.g. submission of the data set, production of output etc) cannot be completed without this data element being present
- R = Required: NHS business processes cannot be delivered without this data element
- O = Optional: the inclusion of this data element is optional as required for local purposes
- X = Not included in the COSDS Message: Cancer Registries obtain the data from another source, or the item is submitted under another Standard and is included here for reference only.
For guidance on submission of the data set, see the Cancer Outcomes and Services Data Set Submission Requirements.
For guidance on the XML Schema constraints, see the Cancer Outcomes and Services Data Set XML Schema Constraints.
| PRE-TREATMENT ASSESSMENT - HEAD AND NECK |
|---|
| To carry pre-treatment assessment details for Head and Neck cancer. One occurrence of this group is permitted. | |
| M/R/O/X | Data Set Data Elements |
| R | OBSERVATION DATE (HEIGHT) |
| R | PERSON HEIGHT IN METRES |
| R | OBSERVATION DATE (WEIGHT) |
| R | PERSON WEIGHT |
| R | CANCER DENTAL ASSESSMENT DATE |
| R | CARE CONTACT DATE (DIETICIAN INITIAL) |
| R | SURGICAL VOICE RESTORATION COMMUNICATION METHOD (PLANNED POST OPERATIVE) |
| POST TREATMENT ASSESSMENT - HEAD AND NECK |
|---|
| To carry post treatment assessment details for Head and Neck cancer. Multiple occurrences of this group are permitted. | |
| M/R/O/X | Data Set Data Elements |
| M | CLINICAL STATUS ASSESSMENT DATE (CANCER) |
| R | PERSON HEIGHT IN METRES |
| R | PERSON WEIGHT |
| R | PRIMARY TUMOUR STATUS |
| R | NODAL STATUS |
| R | METASTATIC STATUS |
| R | SURGICAL VOICE RESTORATION COMMUNICATION METHOD (PRIMARY) |
| R | SPEECH AND LANGUAGE ASSESSMENT DATE |
| PATHOLOGY: GENERAL - HEAD AND NECK |
|---|
| To carry general pathology details for Head and Neck cancer. Multiple occurrences of this group are permitted. | |
| M/R/O/X | Data Set Data Elements |
| M | INVESTIGATION RESULT DATE |
| R | SERVICE REPORT IDENTIFIER |
| PATHOLOGY: VARIOUS - HEAD AND NECK |
|---|
| To carry pathology details for various Head and Neck cancer. One occurrence of this data group is permitted per pathology report where applicable. | |
| M/R/O/X | Data Set Data Elements |
| R | MAXIMUM DEPTH OF INVASION |
| R | BONE INVASION INDICATION CODE |
| R | CARTILAGE INVASION INDICATION CODE |
| R | ANATOMICAL SIDE (NECK DISSECTION) |
| PATHOLOGY: SALIVARY TUMOUR - HEAD AND NECK |
|---|
| To carry pathology salivary tumour details for Head and Neck cancer. One occurrence of this data group is permitted per pathology report where applicable. | |
| M/R/O/X | Data Set Data Elements |
| M | HISTOLOGICAL TUMOUR GRADE (SALIVARY) |
| R | MACROSCOPIC EXTRAGLANDULAR EXTENSION INDICATION CODE |
| PATHOLOGY: GENERAL AND SALIVARY TUMOUR - HEAD AND NECK |
|---|
| To carry general pathology and salivary tumour details for Head and Neck cancer. One occurrence of this data group is permitted per pathology report where applicable. | |
| M/R/O/X | Data Set Data Elements |
| M | ANATOMICAL SIDE (POSITIVE NODES) |
| R | LARGEST METASTASIS (LEFT NECK) |
| R | LARGEST METASTASIS (RIGHT NECK) |
| R | EXTRACAPSULAR SPREAD INDICATION CODE |
Change to Central Return Form: Changed Description
KC53: Adult Screening Programmes: Cervical Screening
This return is in development by the NHS Cancer Screening Programme, therefore the information should not be used.
For the latest version of the form and further details, please see the Health and Social Care Information Centre website.
Part B: Cervical Screening Programme - Number of Women Invited
Part B of KC53 requires age-banded data on the number of women invited for screening, The number invited relates toScreening Test Invitationswith anAPPOINTMENT DATE OFFEREDbetween 1 April and 31 March. This date does not necessarily relate to a due date in the year - e.g. theScreening Testcould be set to take place outside this period. Where a woman is invited on more than one occasion in the year, the last invitation is recorded on KC53.AScreening Test Invitationis anAPPOINTMENTassociated with anAPPOINTMENT OFFERwhere theAPPOINTMENT CLASSIFICATION CODEis National Code 06'Screening Test'.AScreening Testis aCLINICAL INTERVENTIONwhereCLINICAL INTERVENTION TYPEis National Code 28'Screening Test'.Age of woman at 31 March (column 1)Part B of KC53 requires age-banded data on the number of women invited for screening, The number invited relates to Screening Test Invitations with an APPOINTMENT DATE OFFERED between 1 April and 31 March. This date does not necessarily relate to a due date in the year - e.g. the Screening Test could be set to take place outside this period. Where a woman is invited on more than one occasion in the year, the last invitation is recorded on KC53.
A Screening Test Invitation is an APPOINTMENT associated with an APPOINTMENT OFFER for a Screening Test.
A Screening Test is a CLINICAL INTERVENTION where CLINICAL INTERVENTION TYPE is National Code 28 'Screening Test'.Age of woman at 31 March (column 1)
The age bands are derived from the PERSON BIRTH DATE.
Under 20 (line 0001)
20-24 (line 0002)
25-29 (line 0003)
30-34 (line 0004)
35-39 (line 0005)
40-44 (line 0006)
45-49 (line 0007)
50-54 (line 0008)
55-59 (line 0009)
60-64 (line 0010)
65-69 (line 0011)
70-74 (line 0012)
75 & over (line 0013)Call (column 2)
A count of the number of women invited for their first screen i.e. those who have never been screened before. The INVITATION TYPE of the Screening Test Invitation will have the classification First call.
Routine recall (column 3)
A count of the number of women invited for screening in the year as a result of a routine recall for screening. These women will have had a previous negative result and been recalled after the usual interval (3 to 5 years). The INVITATION TYPE of the Screening Test Invitation will have the classification Routine recall.
Surveillance (column 4)
A count of the number of women invited for early screening because of a previous abnormal screening result or following treatment for cervical abnormalities. The INVITATION TYPE of the Screening Test Invitation will have the classification Repeat in less than three years for surveillance.
Abnormality (column 5)
A count of the number of women invited for early screening because their last smear showed some abnormality and a repeat was advised. The INVITATION TYPE of the Screening Test Invitation will have the classification Repeat in less than three years because of abnormality.
Inadequate smear (column 6)
A count of the number of women invited for screening because their last smear was inadequate. The INVITATION TYPE of the Screening Test Invitation will have either the classification Repeat in less than three years because of inadequate smear, or the classification Technical recall (inadequate test).
Target age group (line 0014)
This counts the number of women in the Screening Programme aged between 20 and 64 on 31 March (sum of lines 0002 to 0010).
A Screening Programme is a HEALTH PROGRAMME where the HEALTH PROGRAMME TYPE is National Code 06 'Screening Programme'.
Total all ages (line 9999)
This is the total for all age groups counted in lines 0001 to 0013 for each INVITATION TYPE.
Change to Central Return Form: Changed Description
KC53: Adult Screening Programmes: Cervical Screening
This return is in development by the NHS Cancer Screening Programme, therefore the information should not be used.
For the latest version of the form and further details, please see the Health and Social Care Information Centre website.
Part C1: Cervical Screening Programme - Number of Women Tested - by Age
Part C1 of KC53 requires data on the women screened in the year, by invitation or opportunistically. The number screened relates to Screening Tests with a Screening Test Date between 1 April and 31 March. Where a woman is screened more than once in the year, for whatever reason, her INVITATION TYPE at her first Screening Test Date in the review period is to be recorded.
A Screening Test is a CLINICAL INTERVENTION where the CLINICAL INTERVENTION TYPE is National Code 'Screening Test'. Screening Test Date is the same as attribute ACTIVITY DATE where ACTIVITY DATE TYPE is National Code 'Screening Test'.
Call (column 2)
A count of the number of women screened in the year as a result of a first call for screening within 12 months of the original invitation. These women will not have been screened before. TheINVITATION TYPEof theScreening Test Invitationwill have the classificationFirst call.AScreening Test Invitationis anAPPOINTMENTassociated with anAPPOINTMENT OFFERwhere theAPPOINTMENT CLASSIFICATION CODEis National Code'Screening Test'.Routine recall (column 3)A count of the number of women screened in the year as a result of a first call for screening within 12 months of the original invitation. These women will not have been screened before. The INVITATION TYPE of the Screening Test Invitation will have the classification First call.
A Screening Test Invitation is an APPOINTMENT associated with an APPOINTMENT OFFER for a Screening Test.
Routine recall (column 3)
A count of the number of women screened in the year as a result of a routine recall for screening within 12 months of the recall invitation. These women will have had a previous negative result and been recalled after the usual interval (3 to 5 years). The INVITATION TYPE of the Screening Test Invitation will have the classification Routine recall.
Surveillance (column 4)
A count of the number of women screened in the year as a result of a non-routine recall for screening within 12 months of the recall invitation. The INVITATION TYPE of the Screening Test Invitation will have the classification Repeat in less than 3 years for surveillance.
Abnormality (column 5)
A count of the number of women screened in the year as a result of a non-routine recall for screening within 12 months of the recall invitation. These women will usually have had a recent mildly abnormal smear. The INVITATION TYPE of the Screening Test Invitation will have the classification Repeat in less than 3 years because of abnormality.
Inadequate smear (column 6)
Enter the number of women screened in the year as a result of a technical recall within 12 months of the recall invitation. The INVITATION TYPE of the Screening Test Invitation will have either the classification Repeat in less than 3 years because of inadequate smear or the classification Technical recall (inadequate test).
While recall suspended (column 7)
A count of the number of women screened in the year who were suspended from the call and recall system at the time of their Screening Test Date. These women will have had a Screening Test with the OPPORTUNISTIC SCREENING TYPE classification of 'screened while recall suspended'.
A Screening Test is a CLINICAL INTERVENTION where the CLINICAL INTERVENTION TYPE is National Code 'Screening Test'.
While recall ceased (column 8)
A count of the number of women screened opportunistically in the year who were ceased from the call and recall system at the time of their Screening Test Date. These women will have had a Screening Test with the OPPORTUNISTIC SCREENING TYPE classification of 'screened while recall ceased'.
Not Invited by Programme (column 9)
A count of the number of women screened opportunistically during the year. This includes all women whose Recall Status was "No action", "GP not informed", "GP informed", "ZZZ GP" and those women whose Recall Status was "Final non-responder" where the initial invitation was generated more than 12 months ago. These women will have had a Screening Test with the OPPORTUNISTIC SCREENING TYPE classification of 'not invited by programme'.
Target age group (line 0014)
This counts the number of women in the Screening Programme aged between 20 and 64 on 31 March (sum of lines 0002 to 0010).
Total all women (line 9999)
This is the total for all age groups counted in lines 0001 to 0013 for each INVITATION TYPE or women who have had a Screening Test with the OPPORTUNISTIC SCREENING TYPE recorded.
Change to Central Return Form: Changed Description
KC53: Adult Screening Programmes: Cervical Screening
This return is in development by the NHS Cancer Screening Programme, therefore the information should not be used.
For the latest version of the form and further details, please see the Health and Social Care Information Centre website.
Part C2: Cervical Screening Programme - Number of Women Tested - by Result
Part C2 of KC53 requires data on the women aged 20 - 64 screened in the year, by invitation or opportunistically. The number screened relates to Screening Tests with a Screening Test Date between 1 April and 31 March. Where a woman is screened more than once in the year, for whatever reason, her INVITATION TYPE at her first Screening Test Date in the review period is to be recorded.
A Screening Test is a CLINICAL INTERVENTION where the CLINICAL INTERVENTION TYPE is National Code 'Screening Test'. Screening Test Date is the same as attribute ACTIVITY DATE where the ACTIVITY DATE TYPE is National Code 'Screening Test Date'.
Call (column 2)
A count of the number of women screened in the year as a result of a first call for screening within 12 months of the original invitation. These women will not have been screened before. TheINVITATION TYPEof theScreening Test Invitationwill have the classificationFirst call.AScreening Test Invitationis anAPPOINTMENTassociated with anAPPOINTMENT OFFERwhere theAPPOINTMENT CLASSIFICATION CODEis National Code'Screening Test'.Routine recall (column 3)A count of the number of women screened in the year as a result of a first call for screening within 12 months of the original invitation. These women will not have been screened before. The INVITATION TYPE of the Screening Test Invitation will have the classification First call.
A Screening Test Invitation is an APPOINTMENT associated with an APPOINTMENT OFFER for a Screening Test.
Routine recall (column 3)
A count of the number of women screened in the year as a result of a routine recall for screening within 12 months of the recall invitation. These women will have had a previous negative result and been recalled after the usual interval (3 to 5 years). The INVITATION TYPE of the Screening Test Invitation will have the classification Routine recall.
Surveillance (column 4)
A count of the number of women screened in the year as a result of a non-routine recall for screening within 12 months of the recall invitation. The INVITATION TYPE of the Screening Test Invitation will have the classification Repeat in less than 3 years for surveillance.
Abnormality (column 5)
A count of the number of women screened in the year as a result of a non-routine recall for screening within 12 months of the recall invitation. These women will usually have had a recent mildly abnormal smear. The INVITATION TYPE of the Screening Test Invitation will have the classification Repeat in less than 3 years because of abnormality.
Inadequate smear (column 6)
Enter the number of women screened in the year as a result of a technical recall within 12 months of the recall invitation. The INVITATION TYPE of the Screening Test Invitation will have either the classification Repeat in less than 3 years because of inadequate smear or the classification Technical recall (inadequate test).
While recall suspended (column 7)
A count of the number of women screened in the year who were suspended from the call and recall system at the time of their Screening Test Date. These women will have had a Screening Test with the OPPORTUNISTIC SCREENING TYPE classification of 'screened while recall suspended'.
A Screening Test is a CLINICAL INTERVENTION where the CLINICAL INTERVENTION TYPE is National Code 'Screening Test'.
While recall ceased (column 8)
A count of the number of women screened opportunistically in the year who were ceased from the call and recall system at the time of their Screening Test Date. These women will have had a Screening Test with the OPPORTUNISTIC SCREENING TYPE classification of 'screened while recall ceased'.
Not Invited by Programme (column 9)
A count of the number of women screened opportunistically during the year. This includes all women whose Recall Status was "No action", "GP not informed", "GP informed", "ZZZ GP" and those women whose Recall Status was "Final non-responder" where the initial invitation was generated more than 12 months ago. These women will have had a Screening Test with the OPPORTUNISTIC SCREENING TYPE classification of 'not invited by programme'.
Result of test
This is classified by the following CYTOLOGY RESULT TYPES:
Inadequate (cat. 1) (line 0001)
Negative (cat. 2) (line 0002)
Borderline changes (cat. 8) (line 0003)
Mild dyskaryosis (cat. 3) (line 0004)
Moderate dyskaryosis (cat. 7) (line 0005)
Severe dyskaryosis (cat. 4) (line 0006)
Severe dyskaryosis/?invasive carcinoma (cat. 5) (line 0007)
?Glandular neoplasia (cat. 6) line 0008)Total women tested aged 20-64 (line 9999)
This counts the number of women in the Screening Programme aged between 20 and 64 on 31 March (sum of lines 0001 to 0008).
A Screening Programme is a HEALTH PROGRAMME where the HEALTH PROGRAMME TYPE is National Code 'Screening Programme'.
Change to Central Return Form: Changed Description
KC53: Adult Screening Programmes: Cervical Screening
This return is in development by the NHS Cancer Screening Programme, therefore the information should not be used.
For the latest version of the form and further details, please see the Health and Social Care Information Centre website.
Part C3: Cervical Screening Programme - Number of Tests - by Result
Part C3 of KC53 requires data on all tests in the review period, not limited to the target age group 20 - 64, by invitation or opportunistically. The number screened relates to Screening Tests with a Screening Test Date between 1 April and 31 March. Where a woman is screened more than once in the year, for whatever reason, her INVITATION TYPE at her first Screening Test Date in the review period is to be recorded.
A Screening Test is a CLINICAL INTERVENTION where the CLINICAL INTERVENTION TYPE is National Code 'Screening Test'. Screening Test Date is the same as attribute ACTIVITY DATE where the ACTIVITY DATE TYPE is National Code 'Screening Test Date'.
Call (column 2)
A count of the number of tests in the year as a result of a first call for screening within 12 months of the original invitation. These women will not have been screened before. TheINVITATION TYPEof theScreening Test Invitationwill have the classificationFirst call.AScreening Test Invitationis anAPPOINTMENTassociated with anAPPOINTMENT OFFERwhere theAPPOINTMENT CLASSIFICATION CODEis National Code'Screening Test'.Routine recall (column 3)A count of the number of tests in the year as a result of a first call for screening within 12 months of the original invitation. These women will not have been screened before. The INVITATION TYPE of the Screening Test Invitation will have the classification First call.
A Screening Test Invitation is an APPOINTMENT associated with an APPOINTMENT OFFER for a Screening Test.
Routine recall (column 3)
A count of the number of tests in the year as a result of a routine recall for screening within 12 months of the recall invitation. These women will have had a previous negative result and been recalled after the usual interval (3 to 5 years). The INVITATION TYPE of the Screening Test Invitation will have the classification Routine recall.
Surveillance (column 4)
A count of the number of tests in the year as a result of a non-routine recall for screening within 12 months of the recall invitation. The INVITATION TYPE of the Screening Test Invitation will have the classification Repeat in less than 3 years for surveillance.
Abnormality (column 5)
A count of the number of tests in the year as a result of a non-routine recall for screening within 12 months of the recall invitation. These women will usually have had a recent mildly abnormal smear. The INVITATION TYPE of the Screening Test Invitation will have the classification Repeat in less than 3 years because of abnormality.
Inadequate smear (column 6)
Enter the number of tests in the year as a result of a technical recall within 12 months of the recall invitation. The INVITATION TYPE of the Screening Test Invitation will have either the classification Repeat in less than 3 years because of inadequate smear or the classification Technical recall (inadequate test).
While recall suspended (column 7)
A count of the number of tests in the year of women who were suspended from the call and recall system at the time of their Screening Test Date. These women will have had a Screening Test with the OPPORTUNISTIC SCREENING TYPE classification of 'Screened while recall suspended'
A Screening Test is a CLINICAL INTERVENTION where the CLINICAL INTERVENTION TYPE is National Code 'Screening Test'.
While recall ceased (column 8)
A count of the number of tests in the year of women who were ceased from the call and recall system at the time of their Screening Test Date. These women will have had a Screening Test with the OPPORTUNISTIC SCREENING TYPE classification of 'screened while recall ceased'.
Not Invited by Programme (column 9)
A count of the number of opportunistic tests during the year. This includes all women whose Recall Status was "No action", "GP not informed", "GP informed", "ZZZ GP" and those women whose Recall Status was "Final non-responder" where the initial invitation was generated more than 12 months ago. These women will have had a Screening Test with the OPPORTUNISTIC SCREENING TYPE classification 'not invited by programme'.
Result of test
This is classified by the following CYTOLOGY RESULT TYPES:
Inadequate (cat. 1) (line 0001)
Negative (cat. 2) (line 0002)
Borderline changes (cat. 8) (line 0003)
Mild dyskaryosis (cat. 3) (line 0004)
Moderate dyskaryosis (cat. 7) (line 0005)
Severe dyskaryosis (cat. 4) (line 0006)
Severe dyskaryosis/?invasive carcinoma (cat. 5) (line 0007)
?Glandular neoplasia (cat. 6) line 0008)Total all results (line 9999)
This counts the number of tests in the Screening Programme for all age groups on 31 March (sum of lines 0001 to 0008).
A Screening Programme is a HEALTH PROGRAMME where the HEALTH PROGRAMME TYPE is National Code 'Screening Programme'.
Change to Central Return Form: Changed Description
KH12 - Diagnostic Departments: Radiology, Nuclear Medicine and Medical Physics Imaging and Radiodiagnostic Examinations or Tests in any Part of a Hospital
Contextual OverviewThis return is currently under review. For further information on Annual Imaging and Radiodiagnostics Data, please see the NHS England website.
Contextual Overview
The Department of Health requires a count of all Imaging or Radiodiagnostic Events carried out in hospital DEPARTMENTS. The data are collected to implement a requirement of the Council of the European Union. Council Directives 80/836/Euratom and 96/29/Euratom require the health surveillance of the population, through assessment of radiation dose. Council Directive 97/43/Euratom takes this further by placing a specific requirement in relation to doses from medical exposures.
Information on the return is published annually in the 'Diagnostic Departments' bulletin.
Completing Return KH12 - Imaging and Radiological Examinations or Tests in any Part of a Hospital
The return KH12 requires the following information:
- numbers of imaging and radiodiagnostic examinations or tests by modality;
- numbers of imaging events with intervention (success or failed);
- whether the events take place under the auspices of an imaging department or some other department.
The following are excluded:
- Requests which do not result in the use of an IMAGING MODALITY;
- Imaging performed as part of radiotherapy planning;
- Doppler ultrasound examinations without imaging such as is used for peripheral arterial or venous disease or fetal studies;
- Procedures undertaken as part of the NHS Breast Screening Programme (initial screening and subsequent assessment) but not mammography undertaken for symptomatic women whose referrals are made directly to the imaging department.
Providers should collate data from every DEPARTMENT of the hospital which undertakes imaging or radiodiagnostic examinations or tests under its auspices. Imaging Department includes radiology, nuclear medicine and medical physics departments. Any other DEPARTMENT includes DEPARTMENTS other than radiology, nuclear medicine and medical physics undertaking imaging or radiodiagnostic investigations. This would include for instance an obstetric DEPARTMENT with its own ultrasound apparatus or a cardiology department undertaking imaging/interventional procedures.
Care needs to be taken to avoid double-counting. For example X-rays undertaken in an OPERATING THEATRE should be counted by either the Radiology Department or the surgery department, but not by both.
A KH12 return is required from each NHS Health Care Provider.
The return KH12 relates to ACTIVITY taking place over a 12 month period, between 1 April of one year and 31 March of the following year. The return is made annually and submitted within two months of the end of the year to which it relates, by the end of May at the latest.
Change to Central Return Form: Changed Description
KH12 - Imaging and Radiological Examinations or Tests in any Part of a Hospital
Part 1: Total number of departments on 31 MarchThis return is currently under review. For further information on Annual Imaging and Radiodiagnostics Data, please see the NHS England website.
Part 1: Total number of departments on 31 March
Radiology
Enter the total number of Radiology Departments for the ORGANISATION as of 31 March.
Nuclear Medicine
Enter the total number of nuclear medicine Isotope Procedure Departments for the ORGANISATION as at 31 March.
Medical Physics
Enter the total number of medical physics Isotope Procedure Departments for the ORGANISATION as at 31 March.
Enter the total number of Isotope Procedure Departments where the DEPARTMENT TYPE National Code is 'other' for the ORGANISATION as of 31 March.
Part 2: Number of imaging and radiodiagnostic examinations or tests
Part 2 of the form splits total Imaging or Radiodiagnostic Events by IMAGING MODALITY and introduces the concept of events carried out 'Under auspices of' either an Imaging Department or other DEPARTMENT.
Imaging department
Enter the total number of other Isotope Procedure Departments for the ORGANISATION as at 31 March.
Any other DEPARTMENT which is undertaking imaging or radiodiagnostic investigations.
Modality
The IMAGING MODALITY used during the Imaging or Radiodiagnostic Event.
Part 2(a): Imaging and Radiodiagnostics without intervention
For each IMAGING MODALITY and Imaging Department enter the total number of Imaging or Radiodiagnostic Events having an IMAGING INTERVENTION INDICATOR classification of 'No', that have taken place with ACTIVITY DATES within the period up to March 31.
Part 2(b): Imaging and Radiodiagnostics with intervention (successful or failed)
For each IMAGING MODALITY and Imaging Department, enter the total number of Imaging or Radiodiagnostic Events having an IMAGING INTERVENTION INDICATOR classification of 'Yes', that have taken place with a Clinical Intervention Date within the period up to March 31.
Consistency checks
Before returning the form to the Department of Health, please ensure that:
- Parts 2a and 2b - for all lines: columns (17) = total of columns (10) to (16);
- Parts 2a and 2b - for all columns: line 9 is the sum of lines 1 and 2.
Change to Supporting Information: Changed Description
The ABO System is a classification for CLINICAL INVESTIGATION RESULT ITEM.The ABO System is a CLINICAL CLASSIFICATION.
The ABO System is a system of 4 basic types into which human blood may be classified according to the presence or absence of particular antigens:
- A - Blood group A has A antigens in its red blood cells and anti-B antibodies in its plasma;
- B - Blood group B has B antigens and anti-A antibodies in its plasma;
- O - Blood group O blood has no antigens but both anti-A and anti-B antibodies
- AB - Blood group AB has both A and B antigens but no antibodies, as it would destroy itself.
For further information on the ABO System, see the NHS Choices website.
Change to Supporting Information: Changed Description
Anaesthetic Service is a CLINICAL INTERVENTION.An Anaesthetic Service is an Item Of Service Delivery.
An item of Service Delivery.
The administration of a general anaesthetic, other than in connection with a Maternity Medical Service, which requires the services of a second GENERAL MEDICAL PRACTITIONER, for which a fee may be claimed.An Anaesthetic Service is the administration of a general anaesthetic, other than in connection with a Maternity Medical Service, which requires the services of a second GENERAL MEDICAL PRACTITIONER, for which a fee may be claimed. The fee is payable whether the anaesthetic is administered by the GENERAL MEDICAL PRACTITIONER requesting the services of a second GENERAL MEDICAL PRACTITIONER, or by the second GENERAL MEDICAL PRACTITIONER.
Note that Items of Service Delivery reimbursement cannot be claimed by those GENERAL MEDICAL PRACTITIONERS who hold a Personal Medical Services, as opposed to a General Medical Services, contract.Note that Items Of Service Delivery reimbursement cannot be claimed by those GENERAL MEDICAL PRACTITIONERS who hold a Personal Medical Services, as opposed to a General Medical Services, contract.
References:
Statement of Fees and Allowances Payable to General Medical Practitioners in England and Wales.
Change to Supporting Information: Changed Description
Birth Length is a MEASURED PERSON OBSERVATION.Birth Length is a CLINICAL INVESTIGATION RESULT ITEM.
Birth Length is the length of a baby at birth.
Change to Supporting Information: Changed Description
Birth Weight is a MEASURED PERSON OBSERVATION.Birth Weight is a CLINICAL INVESTIGATION RESULT ITEM.
Birth Weight is the Weight of a baby at birth.
Change to Supporting Information: Changed Description
Blood Pressure is a PERSON PROPERTY.Blood Pressure is a CLINICAL INVESTIGATION RESULT ITEM.
Blood Pressure is the the pressure of the blood within the arteries and is comprised of:Blood Pressure is the pressure of the blood within the arteries and is comprised of:
Change to Supporting Information: Changed Description
A Blood Transfusion is a CLINICAL INTERVENTION.A Blood Transfusion is a CLINICAL INTERVENTION.
A Blood Transfusion is a procedure which is undertaken to replace blood that might be lost for example, during surgery or due to a serious injury. It is also performed when the PATIENT is unable to produce blood properly because of an illness.A Blood Transfusion is a procedure which is undertaken to replace blood that might be lost for example, during surgery or due to a serious injury.
For further information on Blood Transfusions, see the NHS Choices website.
Change to Supporting Information: Changed Description
Body Mass Index (BMI) is a PERSON PROPERTY.Body Mass Index (BMI) is a CLINICAL INVESTIGATION RESULT ITEM.
Body Mass Index is a measure of body fat based on Height and Weight.
For further information on Body Mass Index, see the NHS Choices website.
Change to Supporting Information: Changed Description
Bone Age is a MEASURED PERSON OBSERVATIONBone Age is a CLINICAL INVESTIGATION RESULT ITEM.
Bone Age is the radiological bone age, following an assessment by a radiologist viewing X-rays of the PATIENT's hand and wrist.
Change to Supporting Information: Changed Name, Description
Cervical Glandular Intraepithelial Neoplasia is a CLINICAL INVESTIGATION RESULT ITEM.Cervical Glandular Intra-epithelial Neoplasia is a PATIENT DIAGNOSIS.
Cervical Glandular Intraepithelial Neoplasia is used for PATIENTS with cervical cancer to identify the inner glandular CELLS of the cervix.Cervical Glandular Intra-epithelial Neoplasia is used for PATIENTS with cervical cancer to identify the inner glandular CELLS of the cervix.
Change to Supporting Information: Changed Name, Description
Cervical Intraepithelial Neoplasia is a CANCER STAGING.Cervical Intra-epithelial Neoplasia is a CANCER STAGING.
A Cervical Intraepithelial Neoplasia is divided into grades and is used for PATIENTS with cervical cancer and may be used to describe how abnormal the CELLS are and how much of the cervical TISSUE is involved.A Cervical Intra-epithelial Neoplasia is divided into grades and is used for PATIENTS with cervical cancer and may be used to describe how abnormal the CELLS are and how much of the cervical TISSUE is involved.
For further information on Cervical Intraepithelial Neoplasia, see the National Cancer Institute website.For further information on Cervical Intra-epithelial Neoplasia, see the National Cancer Institute website.
Change to Supporting Information: New Supporting Information
A Clinical Intervention Date and Time is an ACTIVITY DATE TIME.
A Clinical Intervention Date and Time is the Clinical Intervention Date and Clinical Intervention Time of the occurrence of the CLINICAL INTERVENTION.
This supporting information is also known by these names:
| Context | Alias |
|---|---|
| plural | Clinical Intervention Dates |
Change to Supporting Information: New Supporting Information
A Clinical Intervention Time is an ACTIVITY DATE TIME.
A Clinical Intervention Time is the time of the occurrence of the CLINICAL INTERVENTION.
This supporting information is also known by these names:
| Context | Alias |
|---|---|
| plural | Clinical Intervention Times |
Change to Supporting Information: Changed Description
A Clinical Investigation is a CLINICAL INTERVENTION.
A Clinical Investigation is a clinical test or investigation offered to or carried out on a PERSON.
Clinical Investigations may include blood tests for specific antibodies, scans or physical examinations for specific diseases.
A Clinical Investigation may include a Patient Procedure, where it is both diagnostic and therapeutic, for example, certain endoscopic procedures.Change to Supporting Information: Changed Description
Contraceptive Service is a CLINICAL INTERVENTION.A Contraceptive Service is a SERVICE.
An Item of Service Delivery.A Contraceptive Service is the delivery of Contraceptive Services to a PERSON by a GENERAL MEDICAL PRACTITIONER, for which a fee may be claimed.
The delivery of Contraceptive Services to a PERSON by a GENERAL MEDICAL PRACTITIONER, for which a fee may be claimed.
Note that Items of Service Delivery reimbursement cannot be claimed by those GENERAL MEDICAL PRACTITIONERS who hold a Personal Medical Services, as opposed to a General Medical Services, contract.
References:Statement of Fees and Allowances Payable to General Medical Practitioners in England and Wales.
Change to Supporting Information: Changed Description
Dental Haemorrhage Service is a CLINICAL INTERVENTION.A Dental Haemorrhage Service is an Item Of Service Delivery.
An Item of Service Delivery.A Dental Haemorrhage Service is the arresting of a dental haemorrhage or provision of aftercare treatment associated with the dental haemorrhage, delivered by a GENERAL MEDICAL PRACTITIONER to a PATIENT, for which a fee may be claimed.
The arresting of a dental haemorrhage or provision of after care treatment associated with the dental haemorrhage, delivered by a GENERAL MEDICAL PRACTITIONER to a PATIENT, for which a fee may be claimed.Note that Items Of Service Delivery reimbursement cannot be claimed by those GENERAL MEDICAL PRACTITIONERS who hold a Personal Medical Services, as opposed to a General Medical Services, contract.
Note that Items of Service Delivery reimbursement cannot be claimed by those GENERAL MEDICAL PRACTITIONERS who hold a Personal Medical Services, as opposed to a General Medical Services, contract.
References:
Statement of Fees and Allowances Payable to General Medical Practitioners in England and Wales.
Change to Supporting Information: Changed Description
Dental Treatment is a CLINICAL INTERVENTION.
A delivery of dental care of a single DENTAL TREATMENT CLASSIFICATIONS given during a Dental Treatment Contact. This includes all treatment given by community dental services outside Oral Health Programme.Dental Treatment is the delivery of dental care of a single DENTAL TREATMENT CLASSIFICATION given during a Dental Treatment Contact. This includes all treatment given by community dental services outside an Oral Health Programme.
Change to Supporting Information: Changed Description
The Diagnostic waiting times reporting of the monthly waiting times and activity reporting (DM01).
The Diagnostics Waiting Times and Activity Data Set provides definitions to support the national data collections on DIAGNOSTIC TESTS, a key element towards monitoring waits from referral to treatment. ORGANISATIONS responsible for the DIAGNOSTIC TEST activity report the DIAGNOSTIC TEST waiting times and the number of tests completed.
The distinctions between the data set groups are not absolute and some procedures could be collected under more than one of the clinical groupings. A PATIENT waiting for a diagnostic investigation should be counted only once for each test they are waiting for, wherever the test is to be performed and even if there is any additional therapeutic intervention. Each test should be identified by their OPCS Classification of Interventions and Procedures coding where applicable.
The diagnostic investigations are grouped into categories of Imaging, Physiological Measurement and Endoscopy. The data set is for the monthly return covering 15 key DIAGNOSTIC TESTS as below:
IMAGING
- Magnetic Resonance Imaging
- Computer Tomography
- Non-obstetric ultrasound
- Barium Enema
- DEXA Scan (Dual-energy X-ray absorptiometry)
PHYSIOLOGICAL MEASUREMENT
- Audiology - audiological assessments
- Cardiology - echocardiography
- Cardiology - electrophysiology
- Neurophysiology - peripheral neurophysiology
- Respiratory physiology - sleep studies
- Urodynamics - pressures & flows
ENDOSCOPY
- Colonoscopy
- Flexible sigmoidoscopy
- Cystoscopy
- Gastroscopy
How the data set is transmitted
Information is to be submitted onto the Unify2 database that has been developed and maintained by the Department of Health. Full guidance on Unify2 can be found at: Unify2.Further guidance
Change to Supporting Information: Changed Description
The Diagnostic Census of the waiting times for DIAGNOSTIC TEST REQUESTS.
The Diagnostics Waiting Times Census Data Set provides definitions to support the national data collections on DIAGNOSTIC TESTS, a key element towards monitoring waits from referral to treatment. This is a census of DIAGNOSTIC TEST waiting times.
The diagnostic investigations are grouped into categories of Endoscopy, Imaging, Pathology and Physiological Measurement.
The distinctions between these groups are not absolute and some procedures could be collected under more than one of the clinical groupings. A PATIENT waiting for a diagnostic investigation should be counted only once for each test they are waiting for, wherever the test is to be performed and even if there is any additional therapeutic intervention. Each test should be identified by their OPCS Classification of Interventions and Procedures coding where applicable.
This data set is for the census covering 4 main areas of DIAGNOSTIC TESTS as below:
- Part 1 - Endoscopy
- Part 2 - Imaging
- Part 3 - Pathology
- Parts 4 to 11 - Physiological Measurement
Patient level information
Information is to be submitted onto the Unify2 database that has been developed and maintained by the Department of Health. All PATIENTS waiting for a DIAGNOSTIC TEST/procedure funded by the NHS should be included. This includes all referral routes (i.e. whether the PATIENT was referred by a GENERAL PRACTITIONER or by a hospital-based clinician or other route) and also all settings (i.e. Out-Patient Clinic, WARD, Imaging Department, GP Practice, one-stop centres etc.). It is recognised that there will be some overlap between PATIENTS reported on this census and PATIENTS reported in the inpatient and outpatient waiting times returns.How the data set is transmitted
Full guidance on Unify2 can be found at: Unify2.
Further guidance
Guidance on extracting the data sets, including OPCS Classification of Interventions and Procedures, can be found at: Department of Health - Diagnostics.For further guidance on extracting the data sets, see the NHS England website at: Diagnostics Waiting Times and Activity.
Change to Supporting Information: Changed Description
Diastolic Blood Pressure is a MEASURED PERSON OBSERVATION.Diastolic Blood Pressure is part of a Blood Pressure reading, which is a CLINICAL INVESTIGATION RESULT ITEM.
Diastolic Blood Pressure is the reading of a PATIENT's Blood Pressure relaxing between heart beats.
Change to Supporting Information: Changed Name, Description, status to Retired
Dominant Arm is a PERSON PROPERTY This item has been retired from the NHS Data Model and Dictionary.
Dominant Arm is recorded for the purposes of measuring a PATIENT's Blood Pressure.The last live version of this item is available in the September 2013 release of the NHS Data Model and Dictionary.
Blood Pressure differences are common between readings when taken from both PATIENT's arms, and may be slightly higher in the PERSON's Dominant Arm. For example, if the PERSON is left-handed, the left arm may have a slightly higher reading than the right arm.Access to this version can be obtained by emailing information.standards@hscic.gov.uk with "NHS Data Model and Dictionary - Archive Request" in the email subject line.
Change to Supporting Information: Changed Name, Description, status to Retired
- Changed Name from Data_Dictionary.NHS_Business_Definitions.D.Dominant_Arm to Retired.Data_Dictionary.NHS_Business_Definitions.D.Dominant_Arm
- Changed Description
- Retired Dominant Arm
Change to Supporting Information: Changed Description
Dry Weight is a MEASURED PERSON OBSERVATION.Dry Weight is a CLINICAL INVESTIGATION RESULT ITEM.
Dry Weight is a PATIENT's Weight after a Renal Dialysis session when the extra fluid has been removed.
Change to Supporting Information: Changed Description
An Item Of Service Delivery.Emergency Treatment Service is an Item Of Service Delivery.
The delivery of an Emergency Treatment Service by a GENERAL MEDICAL PRACTITIONER, for which a fee may be claimed.Emergency Treatment Service is the delivery of an Emergency Treatment Service by a GENERAL MEDICAL PRACTITIONER, for which a fee may be claimed.
Note that Items of Service Delivery reimbursement cannot be claimed by those GENERAL MEDICAL PRACTITIONERS who hold a Personal Medical Services, as opposed to a General Medical Services, contract.Note that Items Of Service Delivery reimbursement cannot be claimed by those GENERAL MEDICAL PRACTITIONERS who hold a Personal Medical Services, as opposed to a General Medical Services, contract.
References:
Statement of Fees and Allowances Payable to General Medical Practitioners in England and Wales.
Change to Supporting Information: Changed Description
Face To Face Contact Community Care is a CARE CONTACT.
A contact which is made by one or more nurses or community support workers (nursing) with a PATIENT or his/her proxy during a Community Episode. The contact occurs when a PATIENT or their proxy attends a clinic or when the nurse or community support worker (nursing) makes a domiciliary visit to see the PATIENT.Face To Face Contact Community Care is a contact which is made by one or more NURSES or community support workers (nursing) with a PATIENT or his/her proxy during a Community Episode. The contact occurs when a PATIENT or their proxy attends a clinic or when the NURSE or community support worker (nursing) makes a domiciliary visit to see the PATIENT.
A proxy contact is a single occasion involving contact between a proxy and one or more members of a community nurse staff group within a Nursing In The Community Programme.A proxy contact is a single occasion involving contact between a proxy and one or more members of a COMMUNITY NURSE STAFF GROUP within a Nursing In The Community Programme. Contacts with proxies only count if the contact is in lieu of the contact with the PATIENT, and the proxy is able more effectively than the PATIENT to ensure that the specified advice/treatment devised for the PATIENT is followed. This is most likely to be the case where the PATIENT is unable to communicate effectively say for an infant, or for a PERSON who is mentally ill or has learning disabilities.
One or more nurses or community support workers (nursing) in the same or different Nursing In The Community Programmes may be in contact with a PATIENT at the same time.
Contacts should be recorded as follows:
| a. | If one or more NURSES or community support workers (nursing) from the same programme are in contact with one PATIENT at the same time, this should be recorded as one face-to-face contact |
| b. | If one or more NURSES or community support workers (nursing) from different programmes are in contact with one PATIENT at the same time, this should be recorded as one contact for each programme involved |
| c. | For contacts at a Day Care Facility, where repeated contacts may occur during the course of a day, this should be recorded as one contact for each programme involved |
| d. | If two NURSES of different disciplines but both classed in the community nurse staff group other community nurses, such as stomatherapist and a continuing care nurse, are in contact with one PATIENT at the same time, this should be recorded as two face-to-face contacts, one for each discipline |
Group activity, where, for example, general advice is given to several PATIENTS at the same time should not be recorded as Nurse or Midwife Contacts.
A Face To Face Contact Community Care may involve activities attributable to a structured programme, such as the following:A Face To Face Contact Community Care may involve ACTIVITIES attributable to a structured programme, such as the following:
| a. | Screening Test |
| b. | Group Session |
| c. | Health Promotion Other Activity |
| d. | EDUCATIONAL ASSESSMENT |
| e. | Test of Immunity |
| f. | Immunisation Dose Given |
| g. | Face To Face Contact Surveillance |
For such activities they must be recorded as part of the respective structured programmes as well as attributed to the Nursing In The Community Programmes.For such ACTIVITIES they must be recorded as part of the respective structured programmes as well as attributed to the Nursing In The Community Programmes.
If the PATIENT is currently subject to a Mental Health Care Spell and the contact NURSE is also their allocated care programme approach care coordinator then a Face To Face Contact CPA Care Coordinator should also be recorded.
For domiciliary visits, an indication of whether the Face To Face Contact Community Care is the first occasion on which a PATIENT is seen should be recorded as an initial contact.For domiciliary visits, an indication of whether the Face To Face Contact Community Care is the first occasion on which a PATIENT is seen should be recorded as an Initial Contact. A LOCATION type should also be recorded.
Information recorded for a Face To Face Contact Community Care includes:
Change to Supporting Information: Changed Description
Forced Expiratory Volume in 1 second (Absolute Amount) is a MEASURED PERSON OBSERVATION.Forced Expiratory Volume in 1 second (Absolute Amount) is a CLINICAL INVESTIGATION RESULT ITEM.
Forced Expiratory Volume in 1 second (Absolute Amount) is the volume of air that can forcibly be blown out in one second, after full inspiration.
Change to Supporting Information: Changed Description
Forced Expiratory Volume in 1 second (Percentage) is a MEASURED PERSON OBSERVATION.Forced Expiratory Volume in 1 second (Percentage) is a CLINICAL INVESTIGATION RESULT ITEM.
Forced Expiratory Volume in 1 second (Percentage) is the volume of air that can forcibly be blown out in one second, after full inspiration, as a percentage of the predicted value.Forced Expiratory Volume in 1 second (Percentage) is the volume of air that can forcibly be blown out in one second, after full inspiration, as a 'Percentage (%)' of the predicted value.
Change to Supporting Information: Changed Name, Description, status to Retired
Gestation Length In Days is the gestation length of a Fetus Episode recorded as the total number of days.This item has been retired from the NHS Data Model and Dictionary.
The calculation may be:The last live version of this item is available in the September 2013 release of the NHS Data Model and Dictionary.
The number of completed whole weeks of gestation and the remaining number of days of an uncompleted whole week should be calculated from the Gestation Length In Days for input and reporting purposes. Where there is no uncompleted whole week, the number of additional days should be recorded as zero.Access to this version can be obtained by emailing information.standards@hscic.gov.uk with "NHS Data Model and Dictionary - Archive Request" in the email subject line.
For example:
Change to Supporting Information: Changed Name, Description, status to Retired
- Changed Name from Data_Dictionary.NHS_Business_Definitions.G.Gestation_Length_In_Days to Retired.Data_Dictionary.NHS_Business_Definitions.G.Gestation_Length_In_Days
- Changed Description
- Retired Gestation Length In Days
Change to Supporting Information: Changed Description
Hand Grip Strength is a MEASURED PERSON OBSERVATION.Hand Grip Strength is a CLINICAL INVESTIGATION RESULT ITEM.
Hand Grip Strength is the PATIENT's hand grip strength.Hand Grip Strength is the strength of the PATIENT's hand grip.
Change to Supporting Information: Changed Description
HbA1c (Hemoglobin A1c), also known as Glycated Hemoglobin, is a MEASURED PERSON OBSERVATION.HbA1c (Hemoglobin A1c), also known as Glycated Hemoglobin, is a CLINICAL INVESTIGATION RESULT ITEM.
The HbA1c test measures the amount of glucose that is being carried by the red blood cells in the body.The HbA1c test measures the amount of plasma glucose concentration that is being carried by the red blood cells in the body, over a prolonged period of time.
Change to Supporting Information: Changed Description
Head Circumference is a MEASURED PERSON OBSERVATION.Head Circumference is a CLINICAL INVESTIGATION RESULT ITEM.
Head Circumference is the circumference of the PATIENT's head.
Change to Supporting Information: Changed Description
Heart Rate is a MEASURED PERSON OBSERVATION.Heart Rate is a CLINICAL INVESTIGATION RESULT ITEM.
Heart Rate is the number of heart beats per unit of time.
Change to Supporting Information: Changed Description
Height is a MEASURED PERSON OBSERVATION.Height is a CLINICAL INVESTIGATION RESULT ITEM.
Height is the height of a PATIENT on a given date, where the MEASUREMENT VALUE TYPE CODE is 'Metres (m)' or 'Centimetres (cm)'.Height is the height of a PATIENT on a given date, where the UNIT OF MEASUREMENT is 'Metres (m)' or 'Centimetres (cm)'.
Change to Supporting Information: Changed Description
Hip Measurement is a MEASURED PERSON OBSERVATIONHip Measurement is a CLINICAL INVESTIGATION RESULT ITEM.
Hip Measurement is the measurement of the PATIENT's hips.
Change to Supporting Information: Changed Description
A Mantoux Test is a Test Of Immunity.A Mantoux Test is a Screening Test.
The Mantoux Test is used as a Screening Test for tuberculosis infection or disease and as an aid to diagnosis.
Further information about Mantoux Tests can be found on the NHS Immunisation Information website.For further information on Mantoux Tests, see the NHS Choices website.
Change to Supporting Information: Changed Description
An Item Of Service Delivery.A Maternity Medical Service is an Item Of Service Delivery.
The delivery of maternity medical services to a PATIENT by a GENERAL MEDICAL PRACTITIONER, for which a fee may be claimed.A Maternity Medical Service is the delivery of maternity medical services to a PATIENT by a GENERAL MEDICAL PRACTITIONER, for which a fee may be claimed.
Note that Items of Service Delivery reimbursement cannot be claimed by those GENERAL MEDICAL PRACTITIONERS who hold a Personal Medical Services, as opposed to a General Medical Services, contract.Note that Items Of Service Delivery reimbursement cannot be claimed by those GENERAL MEDICAL PRACTITIONERS who hold a Personal Medical Services, as opposed to a General Medical Services, contract.
References:
Statement of Fees and Allowances Payable to General Medical Practitioners in England and Wales.
Change to Supporting Information: Changed Description
Mid Arm Circumference is a MEASURED PERSON OBSERVATION.Mid Arm Circumference is a CLINICAL INVESTIGATION RESULT ITEM.
Mid Arm Circumference is the circumference of the PATIENT's mid arm.
Change to Supporting Information: Changed Description
An Item Of Service Delivery.A Minor Surgery Procedure is an Item Of Service Delivery.
A minor surgical procedure performed on a PATIENT by a GENERAL MEDICAL PRACTITIONER, for which a fee may be claimed.A Minor Surgery Procedure is a surgical procedure performed on a PATIENT by a GENERAL MEDICAL PRACTITIONER, for which a fee may be claimed.
Note that Items of Service Delivery reimbursement cannot be claimed by those GENERAL MEDICAL PRACTITIONERS who hold a Personal Medical Services, as opposed to a General Medical Services, contract.Note that Items Of Service Delivery reimbursement cannot be claimed by those GENERAL MEDICAL PRACTITIONERS who hold a Personal Medical Services, as opposed to a General Medical Services, contract.
References:
Statement of Fees and Allowances Payable to General Medical Practitioners in England and Wales.
Change to Supporting Information: Changed Description
The National Direct Access Audiology Patient Tracking List Data Set collects performance information on a weekly basis, on the Referral To Treatment pathways of PATIENTS who are receiving NHS funded audiology treatment in England, who are not already included in the Referral to Treatment Summary Patient Tracking List Data Set. This includes:
- both analogue and digital hearing aid fittings
- services provided directly by NHS Healthcare Providers and also NHS funded PATIENTS treated via the Independent Sector and third sector providers (collected directly or via Primary Care Trusts)
- both new and existing PATIENTS
- any other PATIENTS attending Audiology services directly
For the purposes of the National Direct Access Audiology Patient Tracking List Data Set, "Direct Access" means PATIENTS who are not referred via Ear, Nose and Throat (ENT) or other hospital CONSULTANT. Any pathways that are subject to the 18 weeks waiting time target for Referral to Treatment are out of scope. For this reason PATIENTS on Ear, Nose and Throat pathways (or pathways from other specialties) are excluded from this central return data set - information on these PATIENTS is available via the Referral to Treatment Summary Patient Tracking List Data Set data collection.
The National Direct Access Audiology Patient Tracking List Data Set is in two parts, as follows:
Parts 1A and 1B: Untreated Patients
Part 1A should be completed for PATIENTS who have not had an ACTIVITY which ends their REFERRAL TO TREATMENT PERIOD (such as first definitive treatment, or a decision not to treat)
AND
who do not have a future APPOINTMENT for an ACTIVITY with an anticipated REFERRAL TO TREATMENT PERIOD STATUS of 30 before the REFERRAL TO TREATMENT PERIOD EXCEEDS 18 WEEKS DATE.
Part 1B should be completed for PATIENTS who have not had an ACTIVITY which ends their REFERRAL TO TREATMENT PERIOD (such as first definitive treatment, or a decision not to treat)
AND
whose REFERRAL TO TREATMENT PERIOD EXCEEDS 18 WEEKS DATE has passed.
Part 2 should be completed for PATIENTS who have a REFERRAL TO TREATMENT PERIOD END DATE within the last 7 days
Full guidance on the completion and submission of the National Direct Access Audiology Waiting Times Data Set is available from the Department of Health website.For further guidance, see NHS England website at: Direct Access Audiology.
The Department of Health document 'Improving Access to Audiology Service in England' can be found at the "Direct Access Audiology Waiting Times and PTL collections section" of the Department of Health website.
Change to Supporting Information: Changed Description
The National Direct Access Audiology Waiting Times Data Set collects performance information on a monthly basis on the Referral To Treatment pathways of PATIENTS who are receiving NHS funded audiology treatment in England, who are not already included in the Referral To Treatment Data Set data collection. This includes:
- both analogue and digital hearing aid fittings
- services provided directly by NHS Healthcare Providers and also NHS funded PATIENTS treated via the Independent Sector and third sector providers (collected directly or via Primary Care Trusts)
- both new and existing PATIENTS
- any other PATIENTS attending Audiology services directly
For the purposes of the National Direct Access Audiology Waiting Times Data Set, "Direct Access" means PATIENTS who are not referred via Ear, Nose and Throat (ENT) specialist or other hospital CONSULTANT. Any pathways that are subject to the 18 week waiting time target for Referral to Treatment are out of scope. For this reason PATIENTS on Ear, Nose and Throat pathways (or pathways from other specialties) are excluded from this central return data set - information on these PATIENTS is available via the Referral To Treatment Data Set data collection.
The National Direct Access Audiology Waiting Times Data Set is in two parts, as follows:
Part 1 - Treated Patients should be completed for PATIENTS who have had an ACTIVITY which ends their REFERRAL TO TREATMENT PERIOD (such as first definitive treatment, or a decision not to treat), within the REPORTING PERIOD.
Part 2 - Untreated Patients should be completed for PATIENTS without a REFERRAL TO TREATMENT PERIOD END DATE within the REPORTING PERIOD.
The Department of Health document 'Improving Access to Audiology Service in England' can be found at the "Direct Access Audiology Waiting Times and PTL collections section" of the Department of Health website.For further guidance, see NHS England website at: Direct Access Audiology.
Change to Supporting Information: Changed Description
ORGANISATIONS such as the Health and Social Care Information Centre, General Medical Council etc which are included in the NHS Data Model and Dictionary.
Change to Supporting Information: Changed Description
Referenced Organisations:American Joint Committee on CancerBritish Association for Paediatric NephrologyBritish Psychological SocietyBritish Renal SocietyBritish Transplantation SocietyCare Quality CommissionCommunity Health Partnership (Scotland)Department for EducationDepartment for Work and PensionsDepartment for Work and Pensions Overseas Healthcare TeamDepartment of HealthEuropean Renal Association (European Dialysis and Transplant Association)Faculty of General Dental Practice (UK)GS1Health and Social Care Information CentreHealth and Wellbeing BoardHealth Education EnglandHealth Research AuthorityHealthwatch EnglandInformation Standards Board for Health and Social CareInternational Federation of Gynecology and ObstetricsInternational Health Terminology Standards Development OrganisationInternational Society of Paediatric OncologyLocal Health Board (Wales)Local HealthwatchNorthern Ireland Local Commissioning GroupMonitorNational Cancer Intelligence NetworkNational Casemix OfficeNational Commissioning GroupNational Institute for Health and Clinical ExcellenceNational Joint RegistryNational Kidney FederationNational Specialised Commissioning GroupNHS Business Services AuthorityNHS EnglandNHS Dental ServicesNHS Prescription ServicesNHS Trust Development AuthorityNHS Wales Informatics ServiceOffice for National StatisticsOfstedOrganisation Data ServicePublic Health EnglandRoyal College of General PractitionersRoyal Pharmaceutical SocietyThe Renal AssociationThe Royal MarsdenUK National Screening CommitteeUK Renal RegistryUK Terminology CentreUnion for International Cancer ControlUnited Kingdom Association of Cancer RegistriesWorld Health Organisation
- Referenced Organisations:
- American Joint Committee on Cancer
- British Association for Paediatric Nephrology
- British Psychological Society
- British Renal Society
- British Transplantation Society
- Care Quality Commission
- Community Health Partnership (Scotland)
- Department for Education
- Department for Work and Pensions
- Department for Work and Pensions Overseas Healthcare Team
- Department of Health
- European Renal Association (European Dialysis and Transplant Association)
- Faculty of General Dental Practice (UK)
- GS1
- Health and Social Care Information Centre
- Health and Wellbeing Board
- Health Education England
- Health Research Authority
- Healthwatch England
- Information Standards Board for Health and Social Care
- International Federation of Gynecology and Obstetrics
- International Health Terminology Standards Development Organisation
- International Society of Paediatric Oncology
- Local Health Board (Wales)
- Local Healthwatch
- Monitor
- National Cancer Intelligence Network
- National Casemix Office
- National Commissioning Group
- National Institute for Health and Clinical Excellence
- National Joint Registry
- National Kidney Federation
- National Specialised Commissioning Group
- NHS Business Services Authority
- NHS England
- NHS Dental Services
- NHS Prescription Services
- NHS Trust Development Authority
- NHS Wales Informatics Service
- Northern Ireland Local Commissioning Group
- Office for National Statistics
- Ofsted
- Organisation Data Service
- Public Health England
- Royal College of General Practitioners
- Royal Pharmaceutical Society
- The Renal Association
- The Royal Marsden
- UK National Screening Committee
- UK Renal Registry
- UK Terminology Centre
- Union for International Cancer Control
- United Kingdom Association of Cancer Registries
- World Health Organisation
Change to Supporting Information: Changed Description
A Palliative Care Episode is an ACTIVITY GROUP.
A Palliative Care Episode is an episode of Palliative Care.
Change to Supporting Information: Changed Description
Patient Procedure is a CLINICAL INTERVENTION.A Patient Procedure is a CLINICAL INTERVENTION.
This is the actual procedure performed on an individual PATIENT at a particular time. A PATIENT may undergo a procedure under the direct personal supervision of a medical or dental practitioner, in pregnancy or childbirth or for the prevention, cure, relief or diagnosis of disease. It should be possible to record at least four procedures for each Consultant Episode (Hospital Provider).A Patient Procedure is a procedure performed on a PATIENT by a CARE PROFESSIONAL.
In the case of an electro-convulsive therapy (ECT) treatment procedure, it comprises the PATIENT being given an anaesthetic for the purpose of electro-convulsive therapy and being administered one or more electric stimuli to the head and should be recorded regardless of whether or not the PATIENT has a convulsion.A Patient Procedure may be carried out:
A Patient Procedure may be carried out as part of a Clinical Investigation, where it is both diagnostic and therapeutic, for example, certain endoscopic procedures.
Change to Supporting Information: Changed Description
Percentage Weight Loss is a CLINICAL INVESTIGATION RESULT ITEM.
Percentage Weight Loss is the percentage of Weight lost by a PATIENT over a specified period.
Percentage Weight Loss is used as an indicator of malnutrition.Percentage Weight Loss may be used as an indicator of malnutrition.
Change to Supporting Information: Changed Description
A Person Stop Smoking Episode is a period of time during which a PERSON attempts to stop smoking as a result of structured stop smoking interventions, delivered by NHS staff or their trained agents. During this time, it is expected that the PERSON will set a SMOKING QUIT DATE.
The Person Stop Smoking Episode:
- starts when the PERSON has consented to a programme of treatment and presents themselves to the Stop Smoking Service for a treatment session and has set a SMOKING QUIT DATE
- ends 28 days after their specified SMOKING QUIT DATE (or within 25 to 42 days of the SMOKING QUIT DATE) when it is confirmed that the PERSON:
- has stopped smoking
- has not been successful or
- are lost to follow up (where the PERSON could not be contacted at four weeks (-3 days or +14 days)).
A PERSON who has participated in an assessment session but failed to attend for treatment should not be counted as having started a Person Stop Smoking Episode.
For further information, see the Department of Health NHS Stop Smoking Services: service and monitoring guidance, October 2007/08.For further information, see the Department of Health part of the gov.uk website at Stop Smoking Service: monitoring and guidance update.
Change to Supporting Information: Changed Description
Post Mortem is a CLINICAL INTERVENTION.A Post Mortem is a CLINICAL INTERVENTION.
A pathology procedure carried out on NHS premises. This should include post mortem examinations of the viable new born fetus but exclude dissection of the pre-viable fetus.A Post Mortem is the pathological examination of a dead body to determine the cause of death.
Change to Supporting Information: Changed Description
Radiology Department is a DEPARTMENT.A Radiology Department is a DEPARTMENT.
A unit based in one or more LOCATIONS which is managed as a separate entity by a CONSULTANT or non-medical scientist of equivalent standing in the MAIN SPECIALTY of radiology which deals with requests for radiological or isotope procedures.A Radiology Department is a unit based in one or more LOCATIONS which is managed as a separate entity by a CONSULTANT or non-medical scientist of equivalent standing in the MAIN SPECIALTY of radiology which deals with requests for radiological or isotope procedures.
A Radiology Department may produce Radiology Investigation Plans, perform Imaging or Radiodiagnostic Events and provide radiology investigation result items.
Change to Supporting Information: Changed Description
Radiotherapy Department is a DEPARTMENT.A Radiotherapy Department is a DEPARTMENT.
A Radiotherapy Department is a facility with rooms in which Radiotherapy Machines are housed and operated. A Radiotherapy Department may be responsible for one or more Radiotherapy Treatment Courses.
Change to Supporting Information: Changed Description
The Rh System is a classification for a CLINICAL INVESTIGATION RESULT ITEM.The Rh System is a CLINICAL CLASSIFICATION.
In addition to the antigens present in the ABO System, red blood cells sometimes have another antigen, a protein called the Rh factor.
- If the Rh factor is present, the PERSON's blood group is RhD positive;
- If the Rh factor is absent, the PERSON is RhD negative.
This means that a PERSON can be one of eight blood groups:
- A RhD positive (A+)
- A RhD negative (A-)
- B RhD positive (B+)
- B RhD negative (B-)
- O RhD positive (O+)
- O RhD negative (O-)
- AB RhD positive (AB+)
- AB RhD negative (AB-).
For further information on the Rh System, see the NHS Choices website.
Change to Supporting Information: Changed Description
Screening Test is a CLINICAL INTERVENTION.A Screening Test is a Clinical Investigation.
A test performed on a PERSON to detect a specific disease or impairment, which may be carried out as part of a Screening Programme. It should be noted that individuals may have to be re-tested shortly after an initial test for technical reasons such as the loss of sample, an unsatisfactory test procedure, or an inconclusive result. The Screening Test is not complete until such re-tests have been satisfactorily completed.A Screening Test is a test performed on a PATIENT to detect a specific disease or impairment, which may be carried out as part of a Screening Programme.
The Screening Test is not complete until such re-tests have been satisfactorily completed.
Change to Supporting Information: Changed Description
Serum Cholesterol Level is a MEASURED PERSON OBSERVATION.Serum Cholesterol Level is a CLINICAL INVESTIGATION RESULT ITEM.
Serum Cholesterol Level is the cholesterol level in a PATIENT's blood.
Change to Supporting Information: Changed Description
Serum Creatinine Level is a MEASURED PERSON OBSERVATION.Serum Creatinine Level is a CLINICAL INVESTIGATION RESULT ITEM.
Serum Creatinine Level is the concentration of creatinine in serum (used as an indicator of renal function).
Change to Supporting Information: Changed Description
A Stop Smoking Service is a SERVICE.
A Stop Smoking Service is a SERVICE set up by a Primary Care Trust to help people give up smoking.
For a SERVICE to be designated as an NHS Stop Smoking Service requires that minimum quality standards should be met. To meet these minimum quality standards all advisers should:
- have received appropriate training for their role,
- carry out the 4 week follow-up promptly, in accordance with the current guidance,
- offer weekly support for at least the first four weeks of a quit attempt,
- attempt to confirm smoking status of all PERSONS self-reporting as having quit at 4 week follow-up by use of a carbon monoxide monitor, except where follow-up is carried out by telephone.
The majority of Stop Smoking Services will operate broadly on the 'Maudsley' model of a clinic providing intensive support, usually on a group therapy basis, to the most dependent smokers. The Stop Smoking Service should also continue to be supplemented by a range of SERVICES in various settings in primary care, secondary care and the community.
Central monitoring of data regarding 52 week follow-up is no longer required however, follow-up at 52 week stage is still recommended as good practice to establish long-term success rates and this information should still be collected locally.
For further information on Stop Smoking Services, see the Department of Health part of the gov.uk website at Stop Smoking Service: monitoring and guidance update.Change to Supporting Information: Changed Description
- Smoking is one of the most significant contributing factors to life expectancy, health inequalities and ill health, particularly cancer and coronary heart disease.
The Department of Health requires information on services provided by NHS Health Care Providers.
- The Stop Smoking Services Quarterly Data Set provides essential information used to monitor the process of achieving the NHS targets to increase life expectancy at birth in England and to monitor the performance of Stop Smoking Services.
Collection and Submission
This return relates to ACTIVITY taking place over a 3 month period. The return is made quarterly and should be submitted by the thirty second working day after the end of the quarter to which it relates.
This data should be submitted for each Primary Care Trust.
The data should be collected on responsible Primary Care Trust basis. The Primary Care Trust's responsible population comprises:
- all PERSONS registered with a GP Practice that forms part of the Primary Care Trust, regardless of where the PERSON is resident, plus any PERSONS not registered with a GP Practice who are resident within the Primary Care Trust's statutory geographical boundary.
- Note that PERSONS resident within the Primary Care Trust's statutory geographical boundary, but registered with a GP Practice that forms part of another Primary Care Trust, are the responsibility of that other Primary Care Trust.
- The only exception to the above rules is where PERSONS receive a Stop Smoking Service at or near their workplace, which may be some distance from their home. For example, a Stop Smoking Service might be provided for commuters at their workplace in a large city. In such circumstances it is likely that people will be drawn from a range of places in the surrounding area e.g. commuters to London who live all around the south-east of England. Where a PERSON is judged to meet these criteria, the Primary Care Trust providing the Stop Smoking Service should include these people in their returns.
- all PERSONS registered with a GP Practice that forms part of the Primary Care Trust, regardless of where the PERSON is resident, plus any PERSONS not registered with a GP Practice who are resident within the Primary Care Trust's statutory geographical boundary.
The information in this Central Return Data Set is transmitted at aggregate level to theHealth and Social Care Information Centre's web based data collection systems athttps://stopsmokingservices.ic.nhs.uk/welcome.aspx. NHS providers enter their data directly.Further information on the NHSStop Smoking Servicesand the monitoring scheme can be found atStop Smoking Services Guidance.The information in this Central Return Data Set is transmitted at aggregate level to the Health and Social Care Information Centre's web based data collection systems at https://stopsmokingservices.hscic.gov.uk/welcome.aspx. NHS providers enter their data directly.
Further information on the NHS Stop Smoking Services and the monitoring guidance can be found on the Department of Health part of the gov.uk website at Stop Smoking Service: monitoring and guidance update.
Synopsis of Data Set Content
The Stop Smoking Services Quarterly Data Set requires the REPORTING PERIOD START DATE and REPORTING PERIOD END DATE for the quarter to which it relates.
The collection is for:
- Part 1A - The number of PERSONS with a Person Stop Smoking Episode setting a SMOKING QUIT DATE and successfully quitting by ETHNIC CATEGORY and PERSON GENDER. Pregnant women should be included but not separately identified.
- Part 1B - The number of PERSONS setting a SMOKING QUIT DATE by AGE BAND AT SMOKING QUIT DATE and PERSON GENDER together with the outcome at 4 week follow-up. Pregnant women should be included but not separately identified.
- Part 1C - The number of PERSONS with a PREGNANCY STATUS of 'Yes' at the time of the SMOKING QUIT DATE and the outcome at 4 week follow-up.
- Part 1D - The number of PERSONS setting a SMOKING QUIT DATE and successful quitters with a FREE PRESCRIPTIONS INDICATOR of 'Entitled to free prescriptions'.
- Part 1E - The number of PERSONS setting a SMOKING QUIT DATE and successful quitters by SOCIO-ECONOMIC CLASSIFICATION
- Part 1F - The number of PERSONS setting a SMOKING QUIT DATE and successful quitters by PHARMACOTHERAPY STOP SMOKING AID RECEIVED
- Part 1G - The number of PERSONS setting a SMOKING QUIT DATE and successful quitters by INTERVENTION SESSION TYPE
- Part 1H - The number of PERSONS setting a SMOKING QUIT DATE and successful quitters by INTERVENTION SETTING
- Part 2A - Financial Allocations for the year by type of allocation. (See STOP SMOKING SERVICE PCT FINANCIAL ALLOCATION and
STOP SMOKING SERVICE OTHER FINANCIAL ALLOCATION.)
Figures should be to the nearest pound. - Part 2B - Cumulative total spend on Stop Smoking Services in the year up to the REPORTING PERIOD END DATE.
(See STOP SMOKING SERVICE CUMULATIVE TOTAL SPEND.)
Parts 2A and 2B should include all monies from whatever source which have been specifically allocated to, or spent on, Stop Smoking Services e.g. additional funding such as Neighbourhood Renewal Funding.
Figures should be to the nearest pound.
- Part 1A - The number of PERSONS with a Person Stop Smoking Episode setting a SMOKING QUIT DATE and successfully quitting by ETHNIC CATEGORY and PERSON GENDER. Pregnant women should be included but not separately identified.
Change to Supporting Information: Changed Description
Systolic Blood Pressure is a MEASURED PERSON OBSERVATION.Systolic Blood Pressure is part of a Blood Pressure reading, which is a CLINICAL INVESTIGATION RESULT ITEM.
Systolic Blood Pressure is the reading of a PATIENT's Blood Pressure at each heart beat.
Change to Supporting Information: Changed Description
Temperature is a MEASURED PERSON OBSERVATION.Temperature is a CLINICAL INVESTIGATION RESULT ITEM.
Temperature is the degree of internal heat of a PATIENT's body.
Change to Supporting Information: Changed Name, Description
Test Of Immunity is a CLINICAL INTERVENTION.Test of Immunity is a Screening Test.
A Test Of Immunity performed as part of an Immunisation Programme for an VACCINE PREVENTABLE DISEASE.A Test of Immunity performed as part of an Immunisation Programme for a VACCINE PREVENTABLE DISEASE.
Change to Supporting Information: Changed Description
Urinary Albumin Level is a MEASURED PERSON OBSERVATION.Urinary Albumin Level is a CLINICAL INVESTIGATION RESULT ITEM.
Urinary Albumin Level is the level of albumin in a urine sample.
Change to Supporting Information: Changed Description
Urine Output is a MEASURED PERSON OBSERVATIONUrine Output is a CLINICAL INVESTIGATION RESULT ITEM.
Urine Output is the output of urine of a PATIENT over a specified period of time (i.e. last hour, last 24 hours).
Change to Supporting Information: Changed Description
An Item Of Service Delivery.A Vaccination Service is an Item Of Service Delivery.
A vaccination or immunisation given to a PERSON in accordance with public policy by or on behalf of a GENERAL MEDICAL PRACTITIONER, for which a fee may be claimed.A Vaccination Service is a vaccination or immunisation given to a PERSON in accordance with public policy, by or on behalf of a GENERAL MEDICAL PRACTITIONER, for which a fee may be claimed.
The applicable payment rate for the Vaccination Service delivered is determined by the combination of VACCINE PREVENTABLE DISEASE and IMMUNISATION COURSE TYPE.
Note that Items of Service Delivery reimbursement cannot be claimed by those GENERAL MEDICAL PRACTITIONERS who hold a Personal Medical Services, as opposed to a General Medical Services, contract.Note that Items Of Service Delivery reimbursement cannot be claimed by those GENERAL MEDICAL PRACTITIONERS who hold a Personal Medical Services, as opposed to a General Medical Services, contract.
References:
Statement of Fees and Allowances Payable to General Medical Practitioners in England and Wales.
Change to Supporting Information: Changed Name, Description, status to Retired
Vasectomy Performed is a CLINICAL INTERVENTION.This item has been retired from the NHS Data Model and Dictionary.
A vasectomy operative procedure performed, excluding those vasectomies performed on NHS PATIENTS using a Hospital Bed.The last live version of this item is available in the September 2013 release of the NHS Data Model and Dictionary.
Change to Supporting Information: Changed Name, Description, status to Retired
- Changed Name from Data_Dictionary.NHS_Business_Definitions.V.Vasectomy_Performed to Retired.Data_Dictionary.NHS_Business_Definitions.V.Vasectomy_Performed
- Changed Description
- Retired Vasectomy Performed
Change to Supporting Information: Changed Description
The purpose of the Venous Thromboembolism Risk Assessment Data Set is to quantify the number of adult PATIENTS (aged 18 and over) admitted to hospital, who are risk assessed for Venous Thromboembolism using the Venous Thromboembolism Risk Assessment Tool to allow appropriate preventative treatment based on guidance from the National Institute for Health and Clinical Excellence.
Collection and submission of the Venous Thromboembolism Risk Assessment Data Set
All providers of NHS funded acute hospital care (including foundation trusts and Independent Providers of acute NHS services) must complete this data collection.
Data on Venous Thromboembolism risk assessments should be uploaded onto UNIFY2 each month no later than 20 working days after the month end. Revisions to the data set before the cut off date are allowed however, revisions made after the cut off date must be made in liaison with the Department of Health.
Full guidance on the Venous Thromboembolism Risk Assessment Tool can be be found at: National Institute for Health and Clinical Excellence website and also The Department of Health Venous Thromboembolism website.For further guidance on the Venous Thromboembolism Risk Assessment Data Set, see the:
Change to Supporting Information: Changed Description
The Venous Thromboembolism Risk Assessment Tool is a type of ASSESSMENT TOOL.
The Venous Thromboembolism Risk Assessment Tool assesses adult PATIENTS (aged 18 or over) admitted to a Hospital Provider, for the risk of Venous Thromboembolism.
For further guidance on the Venous Thromboembolism Risk Assessment Tool, see the:
Change to Supporting Information: Changed Description
Waist Measurement is a MEASURED PERSON OBSERVATION.Waist Measurement is a CLINICAL INVESTIGATION RESULT ITEM.
Waist Measurement is the measurement of a PATIENT's waist.
Change to Supporting Information: Changed Description
Weight is a MEASURED PERSON OBSERVATION.Weight is a CLINICAL INVESTIGATION RESULT ITEM.
Weight is the weight of a PATIENT on a given date, where the MEASUREMENT VALUE TYPE CODE is 'Kilograms (kg)' or 'Grams (g)'.Weight is the weight of a PATIENT on a given date, where the UNIT OF MEASUREMENT is 'Kilograms (kg)' or 'Grams (g)'.
Change to Supporting Information: Changed Name, Description
Release: November 2013
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:- CR1367 (29 November 2013) - ISB 0090 Amd 5/2013 Organisation Data Service – Introduction of New Sub Type Identifier for Private Dental Practices
- CR1359 (29 November 2013) - ISB 0090 Amd 47/2012 Organisation Data Service - Identification Codes for Local Authorities
- DDCN 1407 (Immediate) - DDCN 1407/2013 Clinical Investigations
- DDCN 1415 (Immediate) - DDCN 1415/2013 Area Teams
- DDCN 1411 (Immediate) - DDCN 1411/2013 Update to Supporting Information: SNOMED CT®
Release: September 2013
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:- CR1348 (1 October 2013) - ISB 1597 Amd 35/2012 Breast Screening Programmes Data Set (KC63 and KC62)
- CR1403 (Immediate) - DDCN 1403/2013 Religious or Other Belief System Affiliation
- CR1384 (Immediate) - DDCN 1384/2013 Health and Social Care Information Centre Rebranding of XML Schemas
- CR1397 (Immediate) - DDCN 1397/2013 Retired Main Specialty Codes
Release: July 2013
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:- CR1377 (Immediate) - ISB 0105 Retirement of Accident and Emergency Quarterly Monitoring Data Set (QMAE)
Release: May 2013
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:- CR1363 (Immediate) - ISB 1067 Amd 43/2012 National Workforce Data Set Version 2.6
- CR1382 (Immediate) - DDCN 1382/2013 National Renal Data Set amendment
- CR1381 (Immediate) - DDCN 1381/2013 Healthcare Resource Groups
- CR1235 (1 June 2013) - ISB 1588 Amd 11/2012 Accident and Emergency Clinical Quality Indicators
Release: April 2013
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:- CR1372 (Immediate) - DDCN 1372/2013 Organisation Update: April 2013
- CR1369 (Immediate) - DDCN 1369/2013 Organisation Codes and Organisation Types
- CR1347 (1 April 2013) - ISB 1521 Amd 40/2012 Updates to the Cancer Outcomes and Services Data Set and XML Schema
Release: March 2013
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:- CR1364 (Immediate) - DDCN 1364/2013 Operating Theatre
- CR1335 (1 April 2013) - ISB 1593 Amd 27/2012 Venous Thromboembolism Risk Assessment Data Set
- CR1340 (1 April 2013) - ISB 0090 Amd 37/2012 Organisation Data Service - Non-Legislative Organisations
- CR1321 (1 April 2013) - ISB 0011 Amd 25/2012 Mental Health Minimum Data Set version 4.1
Release: February 2013
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:- CR1336 (Immediate) - DDCN 1336/2013 XML Schema Constraint Pages
- CR1362 (Immediate) - DDCN 1362/2013 Update to Organisations in the NHS Data Model and Dictionary
- CR1246 (Immediate) - DDCN 1246/2013 Guidance for Merging Organisations
- CR1345 (Immediate) - DDCN 1345/2013 e-Government Interoperability Framework (e-GIF) and Government Data Standards Catalogue
- CR1354 (Immediate) - DDCN 1354/2013 Treatment Function Code - Well Babies
Release: December 2012
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:- CR1155 (Immediate) - ISB 1567 Amd 12/2011 National Joint Registry Data Set Version 5
- CR1324 (1 December 2012) - ISB 1067 Amd 23/2012 Workforce Data Set Version 2.5
- CR1196, CR1287 and CR1195 (1 January 2013) - ISB 1521 Amd 64/2010 Cancer Outcomes and Services Data Set, Cancer Outcomes and Services Data Set Message and Retirement of Cancer Registration Data Set and National Cancer Data Set
The following have been incorporated early to allow users to see the changes, but please note that the implementation date is 1 April 2013:
- CR1337 (1 April 2013) - ISB 1072 Amd 30/2012 Update to Child and Adolescent Mental Health Services Secondary Uses Data Set
Release: November 2012
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:- CR1166, CR1167 and CR1306 (1 November 2012) - ISB 0092 Amd-16-2010 Commissioning Data Set Version 6-2, Commissioning Data Set XML Message Version 6-2 and Retirement of CDS 6-0
- CR1305 (1 April 2013) - ISB 0092 Amd 06/2011 Allied Health Professions Referral to Treatment (AHP RTT) Update - CDS 6-2
- CR1286 (1 November 2012) - ISB 0028 Amd 17/2012 Treatment Function Codes Update
- CR1343 (Immediate) - DDCN 1343/2012 Change of name for NHS Commissioning Board Authority
- CR1342 (Immediate) - DDCN 1342/2012 Overseas Visitors Update
- CR1341 (Immediate) - DDCN 1341/2012 Discharge Default Code Descriptions
- CR1323 (Immediate) - National Cancer Waiting Times Monitoring Data Set Update for "Delay Reason To Treatment For Cancer"
CR1323 is a corrigendum to CR1258 (1 July 2012) - ISB 0147 Amd 23/2011 Changes to the National Cancer Waiting Times Monitoring Data Set published in the June 2012 release
The following have been incorporated early to allow users to see the changes, but please note that the implementation date is 1 April 2013:
- CR1231 and CR1288 (1 April 2013) - ISB 1570 Amd 164/2010 HIV and AIDS Reporting Data Set and HIV and AIDS Related Data Set Message
Release: September 2012
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:- CR1103 (Immediate) - ISB 0066 Amd 43/2010 Renal Data Set - Data Item Addition, Changes and Deletions
- CR1334 (Immediate) - DDCN 1334/2012 Psychology Definitions
- CR1331 (Immediate) - DDCN 1331/2012 Activity Date Time Type
- CR1329 (Immediate) - DDCN 1329/2012 Change of name for "Health and Social Care Information Centre"
Release: August 2012
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:- CR1326 (Immediate) - DDCN 1326/2012 Health and Care Professions Council
- CR1241 (Immediate) - DDCN 1241/2012 NHS dictionary of medicines and devices
- CR1292 (Immediate) - ISB 1549 Amd 4/2011 and DDCN 1292/2012 Deprecation and withdrawal of version 3.2 of the Acute Myocardial Infarction Data Set and subsequent retiring of the Data Set from the NHS Data Model and Dictionary
Release: June 2012
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:- CR1314 (Immediate) - DDCN 1314/2012 Reasonable Offer Update
- CR1282 (29 June 2012) - ISB 0090 Amd 36/2011 Independent Sector Healthcare Provider (ISHP) Codes extended for ISHPs and Sites
- CR1258 (1 July 2012) - ISB 0147 Amd 23/2011 Changes to the National Cancer Waiting Times Monitoring Data Set
Release: May 2012
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:- CR1215 (1 June 2012) - ISB 1067 Amd 30/2011 National Workforce Data Set
The following have been incorporated early to allow users to see the changes, but please note that the implementation date is 1 April 2013:
- CR1028 (1 April 2013) - ISB 1069 Amd 14/2012 Children and Young People's Health Services Data Set
- CR1029 (1 April 2013) - ISB 1072 Amd 12/2012 Child and Adolescent Mental Health Services (CAMHS) Data Set
- CR1104 (1 April 2013) - ISB 1513 Amd 13/2012 Maternity Secondary Uses Data Set
Release: March 2012
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:- CR1242 (Immediate) - DDCN 1242/2012 Retirement of Mental Health Minimum Data Set Version 3
- CR1238 and CR1276 (1 April 2012) - ISB 1577 Amd 10/2011 Diagnostic Imaging Data Set and Diagnostic Imaging Data Set Message v 1-0
- CR1290 (Immediate) - DDCN 1290/2012 Data Set Notation
- CR1263 (Immediate) - ISB 0090 Amd 5/2012 Health and Social Care Bill Changes
- CR1255 (31 March 2012) - ISB 1576 Amd 08/2011 Quarterly Bed Availability and Occupancy Data Set
- CR1295 (Immediate) - Retirement of old Commissioning Data Set messages
The Information Standards Board for Health and Social Care have been involved in the redesign and retirement of the old Commissioning Data Set Pages, however a formal Information Standards Notice (ISN) will not be published as there are no changes to data standards.
Release: January 2012
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:- CR1285 (Immediate) - DDCN 1285/2012 Elective Admission Type
- CR1252 (Immediate) - DDCN 1252/2011 Geographic Area Changes
Release: November 2011
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:- CR1264 (Immediate) - ISB 1077 Amd 3/2012 Automatic Identification and Data Capture (AIDC) for Patient Identification Data Set
- CR1274 (Immediate) - DDCN 1274/2011 CDS Prime Recipient Identity Update
The following have been incorporated early to allow users to see the changes, but please note that the implementation date is 1 April 2012:
- CR1265 (1 April 2012) - ISB 1520 Amd 29/2011 Changes to the Improving Access to Psychological Therapies Data Set
Release: October 2011
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:- CR1271 (Immediate) - DDCN 1271/2011 Commissioning Data Set Addressing Grid Update
- CR1268 (Immediate) - DDCN 1268/2011 Sexual Orientation Code
The following has been incorporated early to allow users to see the changes, but please note that the implementation date is 1 April 2012:
- CR1158 and CR1260 (1 April 2012) - ISB 1533 Amd 63/2010 Systemic Anti-Cancer Therapy Data Set and Systemic Anti-Cancer Therapy Data Set Message Schema
The following have been incorporated early to allow users to see the changes, but please note that the implementation date is 1 July 2012:
- CR1270 (1 July 2012) - ISB 1080 Amd 25/2011 Amendments to NHS Health Check Data Set
- CR1250 (1 July 2012) - ISB 1080 Amd 25/2011 NHS Health Checks Data Set Message Schema Version 2.0.0
Release: August 2011
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:- CR1232 (Immediate) - ISB 0034 Amd 26/2006 Systematized Nomenclature of Medicine Clinical Terms (SNOMED CT) - NHS Data Model and Dictionary Overview
- CR1222 (1 April 2012) - ISB 0021 Amd 86/2010 Introduction of the International Classification of Diseases Tenth Revision 4th Edition
- CR1190 (1 September 2011) - ISB 1538 Amd 131/2010 Chlamydia Testing Activity Data Set
- CR1188 (Immediate) - Amd 85/2010 Genitourinary Medicine Clinic Activity Data Set (GUMCAD) Extension to include Enhanced Sexual Health Services (ESHS)
The following data set is initially being introduced for local use only. A future Information Standards Notice will be published to notify providers and system suppliers of the requirement to flow the data set nationally:
- CR1105 (1 April 2012) - ISB 1510 Amd 25/2010 Community Information Data Set
Release: July 2011
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:- CR1249 (Immediate) - DDCN 1249/2011 General Pharmaceutical Council Registration Changes
The following has been incorporated early to allow users to see the changes, but please note that the implementation date is 1 July 2012:
- CR1148 (1 July 2012) - ISB 1080 Amd 129/2010 NHS Health Checks Data Set
Release: June 2011
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:- CR1256 (Immediate) - DDCN 1256/2011 School Definitions
- CR1117 (26 August 2011) - ISB 0090 Amd 94/2010 Organisation Data Service Identification Codes for Local Authorities in England and Wales
- CR1251 (Immediate) - DDCN 1251/2011 Change to the Format/Length of Weekly Hours Worked
- CR1243 (Immediate) - DDCN 1243/2011 National Interim Clinical Imaging Procedure (NICIP) Code Set
Release: April 2011
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:- CR1154 (1 April 2011) - ISB 0011 Amd 87/2010 Mental Health Minimum Data Set Version 4.0
- CR1234 (Immediate) - DDCN 1234/2011 Technology Reference Data Update Distribution Service (TRUD)
- CR1168 (Immediate) - ISB 0097 Amd 140/2010 Genitourinary Medicine Access Monthly Monitoring Data Set Amendments - Removal of Human Immunodeficiency Virus data
The following has been incorporated early to allow users to see the changes, but please note that the implementation date is 1 April 2012:
- CR1050 (1 April 2012) - ISB 1520 Amd 51/2010 Improving Access to Psychological Therapies Data Set
Release: March 2011
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:
- CR1224 (1 April 2011) - ISB 0092 Amd 02/20110 Commissioning Data Set Schema Version 6-1-1
- CR1223 (Immediate) - DDCN 1223/2011 Updates to Family Planning References
- CR1225 (Immediate) - DDCN 1225/2011 Practitioners with Special Interests
- CR1216 (1 April 2011) - ISB 0028 Amd 170/2010 Changes to Treatment Function Codes
- CR1203 (1 April 2011) - ISB 0084 Amd 150/2010 Introduction of OPCS Classification of Interventions and Procedures Version 4.6
Release: January 2011
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:- CR1116 (1 April 2010) - ISB 0003 Amd 79/2010 Immunisation Programmes Activity Data Set (KC50)
- CR1112 (1 April 2010) - ISB 1511 Amd 26/2010 NHS Continuing Healthcare and NHS Funded Nursing Care
- CR1068 (Immediate) - ISB 0133 Amd 161/2010 Change To Central Return: Human Papillomavirus (HPV) Immunisation Programme - Vaccine Monitoring Minimum Data Set
- CR1211 (Immediate) - DDCN 1211/2010 Commissioning Data Set Addressing Grid / Organisation Code (Code of Commissioner) Update
Release: December 2010
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:
- CR1175 (1 April 2011) - ISB 1518 Amd 166/2010 Changes to Sexual and Reproductive Health Activity Data Set
- CR1198 (Immediate) - ISB 1067 Amd 165/2010 National Workforce Data Set
- CR1207 (01 December 2010) - ISB 1573 Amd 168/2010 Mixed-Sex Accommodation
- CR1149 (01 January 2011) - ISB 0139 Amd 99/2010 GUMCAD: Change to Genitourinary (GU) Episode Types
Release: November 2010
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:
- CR1119 (Immediate) - DDCN 1119/2010 Organisation Codes Update
- CR1192 (Immediate) - DDCN 1192/2010 Change of name for "Health Solution Wales"
- CR1199 (Immediate) - DDCN 1199/2010 General Pharmaceutical Council and Royal Pharmaceutical Society of Great Britain Update
- CR1189 (Immediate) - DDCN 1189/2010 National Institute for Health and Clinical Excellence
- CR1187 (Immediate) - DDCN 1187/2010 Introduction of the Department for Education
Release: September 2010
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:
- CR1128 (Immediate) - DDCN 1128/2010 Changes to reporting procedures for Overseas Visitors from the European Economic Area and Switzerland
- CR1173 (Immediate) - DDCN 1173/2010 Care Quality Commission Update
- CR1143 (Immediate) - DDCN 1143/2010 General Pharmaceutical Council
- CR1061 (1 October 2010) - ISB 0092/2010 CDS Type 20: Out-patient: Retirement of Default Codes for Out-patient Procedures
- CR1133 (Immediate) - ISB 00289/2010 National Specialty List
Release: August 2010
- The August 2010 Release introduces the NHS Data Model and Dictionary Help Pages.
Release: July 2010
Information Standards Notices and Data Set Change Notices incorporated into the NHS Data Model and Dictionary:
- CR1134 (Immediate - ISB 1067/2010 Amd 109/2010 National Workforce Data Set
- CR1082 (Immediate) - ISB 0153/2010 Critical Care Minimum Data Set
- CR1121 (Immediate) - DSCN 17/2010 Retirement of Data Standard KC60 Central Return
Release: May 2010
Data Set Change Notices incorporated into the NHS Data Model and Dictionary:
- CR957 (Immediate) - DSCN 19/2010 Central Returns: KA34 Ambulance Services
- CR1069 (Immediate) - Redesign of the Commissioning Data Set Pages
The Information Standards Board for Health and Social Care have been involved in the redesign of the Commissioning Data Set Pages and are satisfied that it meets the requirements of the service, however a formal Information Standards Notice (ISN) will not be published as there are no changes to data standards.
Release: March 2010
Data Set Change Notices incorporated into the NHS Data Model and Dictionary:
- CR1123 (1 April 2010) - DSCN 18/2010 Information Standards Notice (ISN)
- CR1139 (Immediate) - DSCN 16/2010 Person Weight
- CR1130 (Immediate) - DSCN 15/2010 Change of name for "The NHS Information Centre for health and social care"
- CR1013 (April 2010) - DSCN 14/2010 Sexual and Reproductive Health Activity Dataset (SRHAD)
- CR1125 (Immediate) - DSCN 13/2010 NHS Data Model and Dictionary Maintenance Update - Policy Definitions
- CR1122 (Immediate) - DSCN 11/2010 Changes to Family Planning References
Release: January 2010
Data Set Change Notices incorporated into the NHS Data Model and Dictionary:
- CR1115 (Immediate) - DSCN 10/2010 Data Standards: Updating of e-Government Interoperability Framework and Government Data Standards Catalogue References
Release: December 2009
Data Set Change Notices incorporated into the NHS Data Model and Dictionary:
- CR1100 (Immediate) - DSCN 25/2009 NHS Prescription Services Update
- CR1045 (1 December 2009) - DSCN 17/2009 Referral to Treatment Clock Stop Administrative Event
- CR1003 (1 December 2009) - DSCN 16/2009 Commissioning Data Sets: Mandation of 18 Week Referral To Treatment Data Items
Release: November 2009
Data Set Change Notices incorporated into the NHS Data Model and Dictionary:
- CR1113 (Immediate) - DSCN 24/2009 Information Standards Board for Health and Social Care Update
- CR1087 (Immediate) - DSCN 23/2009 Health Professions Council Update
- CR1081 (Immediate) - DSCN 22/2009 Data Standards: NHS Data Model and Dictionary Maintenance Update
- CR1019 (27 November 2009) - DSCN 21/2009 Data Standards: Organisation Data Service (ODS) - Optical Sites and Optical Headquarters
- CR1034 (27 November 2009) - DSCN 20/2009 Data Standards: Organisation Data Service (ODS) - Care Homes in England and Wales and their Headquarters
Release: September 2009
Data Set Change Notices incorporated into the NHS Data Model and Dictionary:
- CR1065 (1 October 2009) - DSCN 15/2009 Data Standards: Organisation Data Service, Local Health Boards
Release: June 2009
Data Set Change Notices incorporated into the NHS Data Model and Dictionary:
- CR1014 (1 June 2009) - DSCN 13/2009 Religious and Other Belief System Affiliation
- CR1074 (Immediate) - DSCN 12/2009 Data Standards: Care Quality Commission
- CR1056 (Immediate) - DSCN 11/2009 Data Standards: NHS Data Model and Dictionary Maintenance Update
- CR1072 (1 December 2009) - DSCN 10/2009 Data Standards: National Radiotherapy Data Set
- CR1073 (Immediate) - DSCN 09/2009 Central Returns: Diagnostic Waiting Times and Activity Data Set
- CR1066 (Immediate) - DSCN 08/2009 Data Standards: NHS Prescription Services and NHS Dental Services
- CR1047 (1 April 2011) - DSCN 07/2009 Data Standards: Diabetic Retinopathy Screening Dataset v3.6
- CR1059 (Immediate) - DSCN 06/2009 Data Standard: National Workforce Data Set v2.1
- CR914 (April 2008 (Retrospective)) - DSCN 05/2009 NHS Stop Smoking Services Quarterly Monitoring Return
- CR899 (Immediate) - DSCN 02/2009 NHS Data Model and Dictionary Maintenance Update
Release: March 2009
Data Set Change Notices incorporated into the NHS Data Model and Dictionary:
- CR1001 (1 April 2009) - DSCN 03/2009 Introduction of Commissioning Data Set Schema Version 6-1 (2008-04-01) and update to Commissioning Data Set Schema Version 6-0 (2008-01-14)
- CR976 (31 March 2009) - DSCN 26/2008 Subject: KP90 - Admissions, Changes in Status and Detentions under the Mental Health Act
- CR1017 (1 April 2009) - DSCN 25/2008 Critical Care Minimum Data Set
- CR1002 (1 April 2009) - DSCN 24/2008 Data Standards: Introduction of Commissioning Dataset Version 6.1
- CR1016 (Immediate) - DSCN 23/2008 4 Byte Version of the Read Codes - Withdrawal
Release: December 2008
Data Set Change Notices incorporated into the NHS Data Model and Dictionary:
- CR1022 (1 January 2009) - DSCN 29/2008 Data Standards: 18 Weeks Referral to Treatment (RTT) Time, Performance Sharing
- CR901 (Immediate) - DSCN 28/2008 Removal of references to EDIFACT and the NHS Wide Clearing Service (NWCS)
- CR843 (1 April 2009) - DSCN 22/2008 Data Standards: National Radiotherapy Data Set
- CR1011 (1 January 2009) - DSCN 20/2008 Data Standards: National Cancer Waiting Times Minimum Data Set
Release: November 2008
Data Set Change Notices incorporated into the NHS Data Model and Dictionary:
- CR1026 (3 November 2008) - DSCN 21/2008 Information Standard: Mental Health Act 2007 Mental Category
Release: August 2008
Data Set Change Notices incorporated into the NHS Data Model and Dictionary:
- CR1018 (Immediate) - DSCN 19/2008 Data Standards: Change of Name for National Administrative Code Services (NACS) to Organisation Data Service (ODS)
- CR956 (1 September 2008) - DSCN 18/2008 Central Return: Human Papillomavirus (HPV) Immunisation Programme, Vaccine Monitoring Minimum Dataset
- CR861 (Immediate) - DSCN 16/2008 Central Return: Hospital and Community Services Complaints and General Practice (including Dental) Complaints - KO41(a) and KO 41(b)
- CR964 (Immediate) - DSCN 14/2008 Central Return: 18 Weeks ‘Adjusted’ Referral to Treatment (RTT) Dataset
- CR965 (Immediate) - DSCN 13/2008 Data Standards: Organisation Data Service (ODS) - Change to the Default Codes Set to Support Changes to GMS Contract
- CR879 (Immediate) - DSCN 12/2008 Data Standards: Quarterly Monitoring: Cancelled Operations Data Set (QMCO)
Release: May 2008
Data Set Change Notices incorporated into the NHS Data Model and Dictionary:
- CR502 (Immediate) - DSCN 10/2008 Data Standards: National Workforce Data Definitions (v2.0)
- CR910 (1 April 2008) - DSCN 08/2008 Data Standards: National Direct Access Audiology Patient Tracking List (PTL) and Waiting Times (WT) data sets
- CR900 (Immediate) - DSCN 07/2008 Data Standards: Inter-Provider Transfer Administrative Minimum Data Set
- CR934 (1 April 2008) - DSCN 06/2008 Data Standards: Mental Health Minimum Data Set (version 3.0)
- CR935 (Immediate) - DSCN 05/2008 Data Standards: 18 Weeks Rules Suite
- CR925 (1 September 2008) - DSCN 04/2008 Genitourinary Medicine Clinic Activity Data Set Change to an Information Standard
- CR942 (1 June 2008) - DSCN 03/2008 General Practice and General Medical Practitioner (GMP) - changes resulting from the introduction of the General Medical Services (GMS) Contract
Release: February 2008
Data Set Change Notices incorporated into the NHS Data Model and Dictionary:
- CR812 (Immediate) - DSCN 01/2008 Central Return: Diagnostics Waiting Times Census Data Set
- CR881 (31 December 2007) - DSCN 42/2007 Central Return: Referral To Treatment Summary Patient Tracking List
- CR904 (Immediate) - DSCN 41/2007 Data Standards: Admission Intended Procedure Update
- CR824 (1 February 2008) - DSCN 39/2007 Data Standards: 48 Hour Genitourinary Medicine Access Monthly Monitoring (GUMAMM)
Release: November 2007
Data Set Change Notices incorporated into the NHS Data Model and Dictionary:
- CR919 (Immediate) - DSCN 38/2007 Data Standards: Mental Health Minimum Data Set Schema
- CR814 (1 April 2008) - DSCN 37/2007 Data Standards: Introduction of Mental Health Minimum Data Set version 2.1
- CR930 (31 December 2007) - DSCN 35/2007 Data Standards: A correction to the version 6 Commissioning Data Set schema
- CR834 (Immediate) - DSCN 34/2007 Data Standards: Referral Request Received Date
- CR875 (Immediate) - DSCN 33/2007 Data Standards: National Administrative Codes Service: Introduction of codes for the new Pan SHAs
- CR880 (Immediate) - DSCN 29/2007 Data Standards: Amendments to Doctor Index Number (DIN) Description
Release: August 2007
Data Set Change Notices incorporated into the NHS Data Model and Dictionary:
- CR845 (Immediate) - DSCN 28/2007 Data Standards: Treatment Function Code (Referral to Treatment Period)
- CR831 (1 October 2007) - DSCN 27/2007 Data Standards: Update to Commissioning Data Set XML Schema v5
- CR825 (1 October 2007) - DSCN 16/2007 Data Standards: Source of Referral for Outpatients (18 Weeks)
Release: June 2007
Data Set Change Notices incorporated into the NHS Data Model and Dictionary:
- CR799 (31 December 2007) - DSCN 18/2007 Data Standards: Introduction of Commissioning Data Set Version 6
- CR833 (Immediate) - DSCN 17/2007 Data Standards: Introduction of Commissioning Data Set validation table
- CR801 (Immediate) - DSCN 15/2007 Data Standards: Cover of Vaccination Evaluated Rapidly (COVER) Return
Release: May 2007
Data Set Change Notices incorporated into the NHS Data Model and Dictionary:
- CR800 (31 December 2007) - DSCN 14/2007 Commissioning Data Set Schema Version 6-0
- CR856 (1 October 2007) - DSCN 13/2007 Data Standards: Discharge Ready Date
- CR869 (Immediate) - DSCN 12/2007 Data Standards: Update to Clinical Coding Introduction
- CR827 (1 October 2007) - DSCN 09/2007 Data Standards: Earliest Reasonable Offer Date
- CR817 (1 October 2007) - DSCN 08/2007 Data Standards: Introduction of Age into Commissioning Data Sets
- CR849 (May 2007) - DSCN 07/2007 National Administrative Codes Service: Introduction of new identification codes for Dental Consultants
- CR822 (Immediate) - DSCN 06/2007 Data Standards: Update to Organisation Codes
- CR850 (Immediate) - DSCN 05/2007 National Administrative Codes Service: Amendments to Default Codes
- CR786 (1 April 2007) - DSCN 04/2007 Quarterly Monitoring Accident and Emergency Services (QMAE) Central Return
Release: February 2007
Data Set Change Notices incorporated into the NHS Data Model and Dictionary:
- CR811 (Immediate) - DSCN 03/2007 Diagnostic Waiting Times and Activity
- CR826 (1 October 2007) - DSCN 02/2007 Extension of Treatment Function to Support the Measurement of 18 Week Referral to Treatment Periods
- CR813 (1 April 2007) - DSCN 01/2007 Paediatric Critical Care Minimum Data Set
- CR768 (1 January 2007) - DSCN 18/2006 Changes to the NHS Data Dictionary to support the measurement of 18 week referral to treatment periods
- CR798 (6 November 2006) - DSCN 19/2006 Commissioning Data Set (CDS) Version 5 XML Message Schema
- CR776 (1 October 2006) - DSCN 05/2006 Data Standards: Accident and Emergency Enhancements to Investigation and Treatment Codes
Release: September 2006
Data Set Change Notices incorporated into the NHS Data Model and Dictionary:
- CR795 (31 October 2006) - DSCN 22/2006 Organisation Codes / Organisation Site Codes
- CR792 (1 April 2007) - DSCN 15/2006 Neonatal Critical Care
- CR719 (1 April 2006) - DSCN 09/2006 Measuring and Recording of Waiting Times
- CR791 (1 April 2007) - DSCN 13/2006 Priority Type
- CR774 (1 September 2006) - DSCN 12/2006 Person Marital Status
Release: May 2006
Data Set Change Notices incorporated into the NHS Data Model and Dictionary:
- CR764 (1 April 2006) - DSCN 08/2006 Diagnostics waiting times and activity
- Correction to menu structure to include Critical Care Minimum Data Set
Release: April 2006
Data Set Change Notices incorporated into the NHS Data Model and Dictionary:
- CR608 (1 October 2006) - DSCN 07/2006 Introduction of Commissioning Data Set Version 5 and its associated XML schema into the NHS Data Dictionary.
- CR756 (1 September 2005) - DSCN 19/2005 PbR Commissioning for Out of Area Treatments (OATs) and Charge-Exempt Overseas Visitors
- CR724 (1 April 2006) - DSCN 13/2005 Critical Care Minimum Data Set
- CR754 (1 April 2006) - DSCN 17/2005 Treatment Function and Main Specialty Code Revisions
- CR763 (1 April 2006) - DSCN 20/2005 New Treatment Functions for therapy services and anticoagulant service
- CR767 (Immediate) - DSCN 02/2006 Referral Request Received Date
- CR690 (1 September 2005) - DSCN 16/2005 Marital Status
Release: August 2005
Data Set Change Notices incorporated into the NHS Data Model and Dictionary:
- CR555 (1 April 2005) - DSCN 11/2005 Data Standards: COVER - Hepatitis B immunisation for babies
- CR715 (Immediate) - DSCN 10/2005 Data Standards: Treatment Function Codes - correction and clarification of names and descriptions
- CR706 (1 April 2005) - DSCN 09/2005 Data Standards: Cancer Registration Data Set
- CR691 (1 July 2005) - DSCN 06/2005 Data Standards: NSCAG Commissioner Code
For all Information Standards Notices and Data Set Change Notices, see the Information Standards Board for Health and Social Care Website.
Change to Class: Changed Attributes
| A and E INCIDENT LOCATION TYPE | ||
| A and E PATIENT GROUP | ||
| ACTIVITY GROUP TYPE | ||
| ADMISSION METHOD | ||
| BABY FIRST FEED BREAST MILK STATUS | ||
| BREASTFEEDING STATUS | ||
| CANCER OR SYMPTOMATIC BREAST REFERRAL PATIENT STATUS | ||
| CANCER REFERRAL TO TREATMENT PERIOD START DATE | ||
| CANCER SCREENING STATUS | ||
| CANCER TREATMENT INTENT | ||
| CANCER TREATMENT PERIOD START DATE | ||
| CARE PROGRAMME APPROACH LEVEL | ||
| CHILDREN TEENAGERS AND YOUNG ADULTS AGE CATEGORY | ||
| DELIVERY FACILITIES ONLY USED | ||
| DELIVERY PLACE CHANGE REASON | ||
| DISCHARGE DESTINATION | ||
| DISCHARGED TO HOSPITAL AT HOME SERVICE INDICATOR | ||
| DISCHARGE FROM MENTAL HEALTH SERVICE REASON | ||
| DISCHARGE METHOD | ||
| FIRST REGULAR DAY OR NIGHT ADMISSION | ||
| FULL POSTNATAL EXAMINATION DATE | ||
| GENERAL DENTAL SERVICE INDICATOR | ||
| IMPROVING ACCESS TO PSYCHOLOGICAL THERAPIES CARE SPELL END CODE | ||
| INCIDENT TYPE | ||
| INTRAVESICAL CHEMOTHERAPY RECEIVED INDICATOR | ||
| INTRAVESICAL IMMUNOTHERAPY RECEIVED INDICATOR | ||
| KEY WORKER SEEN INDICATOR | ||
| LENGTH OF STAY ADJUSTMENT | ||
| LENGTH OF STAY ADJUSTMENT REASON | ||
| MONITORING INTENT | ||
| NON SMOKING CONFIRMATION STATUS AT 4 WEEKS | ||
| OUTCOME AT 4 WEEK FOLLOW-UP | ||
| PAEDIATRIC NEPHROLOGY REGISTRY STATUS CODE | ||
| PALLIATIVE CARE SPECIALIST SEEN INDICATOR | ||
| PALLIATIVE TREATMENT REASON CODE FOR UPPER GASTROINTESTINAL | ||
| PHARMACOTHERAPY STOP SMOKING AID RECEIVED | ||
| PREGNANCY OUTCOME CODE | ||
| PREGNANCY TOTAL PREVIOUS LOSSES LESS THAN 24 WEEKS | ||
| PREVIOUS NEGATIVE HIV TEST IN UNITED KINGDOM INDICATOR | ||
| RADIOTHERAPY INTENT | ||
| RENAL DIALYSIS SCHEDULE TYPE | ||
| SMOKING QUIT DATE | ||
| SOURCE OF ADMISSION | ||
| SUPERVISED COMMUNITY TREATMENT END REASON | ||
| SUPERVISION REGISTER RISK | ||
| TELEPHONE CONTACT INDICATOR | ||
| TREATMENT START DATE FOR CANCER | ||
| WARD STAY TERMINATION REASON |
Change to Class: Changed Description
A subtype of PERSON PROPERTY.
Observations made regarding a PERSON. These observations do not include information about a treatment or intervention. CATEGORY VALUED PERSON OBSERVATIONS do not include information about a treatment or intervention.
CATEGORY VALUED PERSON OBSERVATION TYPE provides coded classifications of observations about a PERSON.
Note: MEASURED PERSON OBSERVATION allows for recording of measurements about a PERSON and OTHER PERSON OBSERVATION is where the PERSON states, for example, when they first experienced symptoms, the number of days on which alcohol has been consumed etc.Note: CLINICAL INVESTIGATION RESULT ITEM captures measurements about a PERSON and OTHER PERSON OBSERVATION is where the PERSON states, for example, when they first experienced symptoms, the number of days on which alcohol has been consumed etc.
Change to Class: Changed Attributes, Description
A result of a single Clinical Investigation including all essential or useful relevant data.The result of a Clinical Investigation.
Note: A CLINICAL INVESTIGATION RESULT ITEM includes all useful information in connection with an investigation result (e.g. numerical value, date and time of Clinical Investigation etc.); this corresponds to what is normally called a 'line' on a paper report.CLINICAL INVESTIGATION RESULT ITEM TYPE provides a list of CLINICAL INVESTIGATION RESULT ITEMS.
References:
The Version 1.0 Trial NHS Standard EDIFACT Messages for Radiology Requests and Reports, 14.3.95
The Version 1.0 Trial NHS Standard EDIFACT Messages for GP-Hospital Communications - 17.5.95
Change to Class: Changed Attributes, Description
| K | INVESTIGATION RESULT DATE | |
| K | INVESTIGATION RESULT TIME | |
| ABNORMALITY DETECTED INDICATOR | ||
| ALBUMINURIA STAGE | ||
| ALK 1 STATUS | ||
| ANKLE DORSIFLEXION CODE | ||
| ANKLE PLANTARFLEXION CODE | ||
| ARITHMETIC COMPARATOR | ||
| BIOPSY REFERRAL OUTCOME | ||
| BREAST BIOPSY REFERRAL OUTCOME | ||
| BREAST CANCER HISTOLOGICAL TYPE | ||
| BREAST SCREENING MAMMOGRAPHY OUTCOME CODE | ||
| CANCER VASCULAR OR LYMPHATIC INVASION | ||
| CERVICAL GLANDULAR INTRAEPITHELIAL NEOPLASIA PRESENCE AND GRADE | ||
| CERVICAL NODE STATUS | ||
| CERVICAL SMEAR EXAMINED DATE | ||
| CHLAMYDIA TEST RESULT | ||
| CLINICAL ASSESSMENT RESULT CODE FOR BREAST CANCER | ||
| CLINICAL INVESTIGATION ITEM TYPE | ||
| CLINICAL INVESTIGATION ITEM UNIT OF MEASURE | ||
| CLINICAL INVESTIGATION RESULT CODE FOR RENAL CARE | ||
| CLINICAL INVESTIGATION RESULT CODE FOR RENAL TRANSPLANT | ||
| CLINICAL INVESTIGATION RESULT VALUE | ||
| CYTOGENETIC ANALYSIS CODE | ||
| CYTOGENETIC PRESENCE TYPE FOR RHABDOMYOSARCOMA | ||
| CYTOGENETIC RISK CODE | ||
| CYTOLOGY RESULT TYPE | ||
| CYTOLOGY SMEAR REASON | ||
| DEGREES OF FIXED FLEXION DEFORMITY | ||
| DEGREES OF FLEXION RANGE | ||
| DETRUSOR MUSCLE PRESENCE INDICATION CODE | ||
| DEVIATING RESULT INDICATOR | ||
| DIPSTICK TEST RESULT CODE | ||
| EPIDERMAL GROWTH FACTOR RECEPTOR MUTATIONAL STATUS | ||
| EXCISION MARGIN | ||
| GENETIC CONFIRMATION INDICATOR | ||
| GRADE OF DIFFERENTIATION | ||
| HbA1C ASSAY MEASUREMENT METHOD | ||
| HEPATOMEGALY INDICATOR | ||
| HORMONE EXPRESSION TYPE | ||
| INVASIVE CANCER SPECIAL TYPE INDICATOR | ||
| INVESTIGATION EXAMINATION RESULT CODE | ||
| INVESTIGATION HAEMOGLOBINOPATHY RESULT CODE | ||
| INVESTIGATION RESULT STATUS CODE | ||
| INVESTIGATION RESULT TEXT | ||
| INVESTIGATION RISK RATIO RESULT CODE | ||
| INVESTIGATION RUBELLA RESULT INDICATOR | ||
| INVESTIGATION SENSITISED RESULT INDICATOR | ||
| KARYOTYPE TEST OUTCOME | ||
| LACTATE DEHYDROGENASE LEVEL | ||
| LYMPH NODE STATUS | ||
| MAMMOGRAM RESULT CODE | ||
| METASTASIS EXTENT CODE | ||
| NEWBORN BLOOD SPOT TEST OUTCOME STATUS CODE | ||
| NEWBORN HEARING SCREENING OUTCOME | ||
| NUMBER OF FETUSES | ||
| NUMERICAL VALUE | ||
| PATHOLOGICAL RISK CLASSIFICATION CODE AFTER NEPHRECTOMY | ||
| PATHOLOGICAL RISK CLASSIFICATION CODE AFTER PREOPERATIVE CHEMOTHERAPY | ||
| PERSON BLOOD GROUP | ||
| PERSON RHESUS FACTOR | ||
| PREOPERATIVE THERAPY RESPONSE TYPE | ||
| RADIOLOGICAL RESULT VERIFIED DATE | ||
| RADIOLOGICAL RESULT VERIFIED TIME | ||
| RESULT ITEM STATUS | ||
| S CATEGORY CODE | ||
| SCREENING TEST RESULT | ||
| SERUM CALCIUM CONCENTRATION CORRECTION CODE | ||
| SMEAR INFECTION TYPE | ||
| SPECIMEN NATURE | ||
| SPLEEN BELOW COSTAL MARGIN | ||
| SPLENOMEGALY INDICATOR | ||
| SUBTALAR JOINT MOVEMENT CODE | ||
| TIBIA HINDFOOT ALIGNMENT CODE | ||
| TUMOUR NECROSIS | ||
| ULTRASOUND RESULT CODE FOR BREAST CANCER |
Change to Class: Changed Attributes, Supertype, Name, Description, status to Retired
A subtype of PERSON PROPERTY.This item has been retired from the NHS Data Model and Dictionary.
MEASURED PERSON OBSERVATION allows for recording of measurements about a PERSON.The last live version of this item is available in the September 2013 release of the NHS Data Model and Dictionary.
MEASURED PERSON OBSERVATION TYPE CODE provides a list of MEASURED PERSON OBSERVATIONS.Access to this version can be obtained by emailing information.standards@hscic.gov.uk with "NHS Data Model and Dictionary - Archive Request" in the email subject line.
Note: CATEGORY VALUED PERSON OBSERVATION allows coded classifications of observations about a PERSON and OTHER PERSON OBSERVATION is where the PERSON states, for example, when they first experienced symptoms, the number of days on which alcohol has been consumed etc.
Change to Class: Changed Attributes, Supertype, Name, Description, status to Retired
Change to Class: Changed Attributes, Supertype, Name, Description, status to Retired
- Changed Attributes
- Changed Supertype from Data_Dictionary.Classes.P.PERSON_PROPERTY to null
- Changed Name from Data_Dictionary.Classes.M.MEASURED_PERSON_OBSERVATION to Retired.Data_Dictionary.Classes.M.MEASURED_PERSON_OBSERVATION
- Changed Description
- Retired MEASURED PERSON OBSERVATION
Change to Class: Changed Attributes, Relationships, Name, Description, status to Retired
Identifies the type of MEASURED PERSON OBSERVATION being recorded, for example Height and Weight.This item has been retired from the NHS Data Model and Dictionary.
Access to this version can be obtained by emailing information.standards@hscic.gov.uk with "NHS Data Model and Dictionary - Archive Request" in the email subject line.
Change to Class: Changed Attributes, Relationships, Name, Description, status to Retired
Change to Class: Changed Attributes, Relationships, Name, Description, status to Retired
Change to Class: Changed Attributes, Relationships, Name, Description, status to Retired
- Changed Attributes
- Changed Relationships
- Changed Name from Data_Dictionary.Classes.M.MEASURED_PERSON_OBSERVATION_TYPE to Retired.Data_Dictionary.Classes.M.MEASURED_PERSON_OBSERVATION_TYPE
- Changed Description
- Retired MEASURED PERSON OBSERVATION TYPE
Change to Class: Changed Attributes, Relationships, Name, Description, status to Retired
Identifies the unit of measurement used to record a MEASURED PERSON OBSERVATION, for example Metres, kilograms etc.This item has been retired from the NHS Data Model and Dictionary.
Access to this version can be obtained by emailing information.standards@hscic.gov.uk with "NHS Data Model and Dictionary - Archive Request" in the email subject line.
Change to Class: Changed Attributes, Relationships, Name, Description, status to Retired
Change to Class: Changed Attributes, Relationships, Name, Description, status to Retired
Change to Class: Changed Attributes, Relationships, Name, Description, status to Retired
- Changed Attributes
- Changed Relationships
- Changed Name from Data_Dictionary.Classes.M.MEASUREMENT_VALUE_TYPE to Retired.Data_Dictionary.Classes.M.MEASUREMENT_VALUE_TYPE
- Changed Description
- Retired MEASUREMENT VALUE TYPE
Change to Class: Changed Name, Description, status to Retired
Identifies the valid MEASUREMENT VALUE TYPE that is used for a particular MEASURED PERSON OBSERVATION TYPE. Examples would be Height measured in metres or Weight measured in kilograms.This item has been retired from the NHS Data Model and Dictionary.
Access to this version can be obtained by emailing information.standards@hscic.gov.uk with "NHS Data Model and Dictionary - Archive Request" in the email subject line.
Change to Class: Changed Name, Description, status to Retired
- Changed Name from Data_Dictionary.Classes.O.OBSERVATION_MEASUREMENT_VALIDATION to Retired.Data_Dictionary.Classes.O.OBSERVATION_MEASUREMENT_VALIDATION
- Changed Description
- Retired OBSERVATION MEASUREMENT VALIDATION
Change to Class: Changed Attributes
| K | ORGANISATION BED AVAILABILITY START DATE | |
| AVAILABLE BED DAYS NHS PATIENTS | ||
| AVAILABLE BED DAYS NON-NHS PATIENTS | ||
| ORGANISATION BED AVAILABILITY END DATE |
Change to Class: Changed Attributes
| K | ORGANISATION SITE BED OCCUPANCY START DATE | |
| OCCUPIED BED DAYS NHS PATIENTS | ||
| OCCUPIED BED DAYS NON-NHS PATIENTS | ||
| ORGANISATION SITE BED OCCUPANCY END DATE |
Change to Class: Changed Description
A subtype of PERSON PROPERTY.
Observations made by a PERSON which are not coded or measured.
These observations do not include information about a treatment or intervention. These observations may be where the PERSON states, for example, when they first experienced symptoms, the number of days on which alcohol has been consumed etc.
Note: CATEGORY VALUED PERSON OBSERVATION allows coded classifications of observations about a PERSON and MEASURED PERSON OBSERVATION allows for the recording of measurements about a PERSON.Note: CATEGORY VALUED PERSON OBSERVATION allows coded classifications of observations about a PERSON and CLINICAL INVESTIGATION RESULT ITEM captures measurements about a PERSON.
Change to Class: Changed Attributes, Description
A PERSON PROPERTY is a condition or state associated with a PERSON.
PERSON PROPERTIES are collected as a result of an ACTIVITY.
PERSON PROPERTIES for a PATIENT do not include information about a treatment or intervention.
- The PERSON PROPERTY may be a clinical diagnosis
- PERSON PROPERTIES may be recorded during, or as a result of, a course of treatment.
Subtypes of PERSON PROPERTY include:
Change to Class: Changed Attributes, Description
| K | PERSON PROPERTY IDENTIFIER | |
| ATTEMPTED SUICIDE WITH INTENT INDICATOR | ||
| DOMINANT ARM CODE | ||
| FAMILIAL CANCER SYNDROME INDICATOR | ||
| FREE PRESCRIPTIONS INDICATOR | ||
| LAST MENSTRUAL PERIOD DATE | ||
| PERSON PROPERTY EFFECTIVE DATE | ||
| PERSON PROPERTY EFFECTIVE END DATE | ||
| PERSON PROPERTY EFFECTIVE END TIME | ||
| PERSON PROPERTY EFFECTIVE TIME | ||
| PERSON PROPERTY OBSERVED DATE | ||
| PERSON PROPERTY OBSERVED TIME | ||
| PERSON PROPERTY RECORDED DATE | ||
| PERSON PROPERTY RECORDED TIME | ||
| PREGNANCY STATUS | ||
| SURGICAL VOICE RESTORATION COMMUNICATION METHOD FOR PLANNED POST OPERATIVE | ||
| SURGICAL VOICE RESTORATION COMMUNICATION METHOD FOR PRIMARY | ||
| YOUNG CARER INDICATOR |
Change to Class: Changed Attributes
| ANATOMICAL AREA | ||
| ANATOMICAL SIDE | ||
| ANATOMICAL SIDE FOR IMAGING | ||
| ANATOMICAL SITE | ||
| HYDRONEPHROSIS CODE | ||
| PERSON PROPERTY QUALIFIER IDENTIFIER | ||
| PRIMARY EXTRANODAL SITE | ||
| RADIOTHERAPY TREATMENT REGION | ||
Change to Class: Changed Attributes
| APGAR SCORE 1 MINUTE | ||
| APGAR SCORE 5 MINUTE | ||
| BCG ADMINISTERED | ||
| BIRTH HEAD CIRCUMFERENCE | ||
| BIRTH ORDER | ||
| DELIVERY METHOD | ||
| DELIVERY PLACE TYPE | ||
| DELIVERY TIME | ||
| EXAMINATION OF HIPS | ||
| FOLLOW UP CARE | ||
| GESTATION LENGTH IN DAYS | ||
| GESTATION LENGTH IN WEEKS | ||
| LIVE OR STILL BIRTH | ||
| METABOLIC SCREENING | ||
| NUMBER OF BABIES IDENTIFIER | ||
| PRESENCE OF JAUNDICE | ||
| PRESENTATION AT ONSET OF LABOUR | ||
| RESUSCITATION METHOD DRUGS | ||
| RESUSCITATION METHOD POSITIVE PRESSURE | ||
| STATUS OF PERSON CONDUCTING DELIVERY |
Change to Class: New Class
The unit of measurement.
This could be, for example, for a CLINICAL INVESTIGATION RESULT ITEM, PRESCRIBED ITEM, PERSON PROPERTY.
This class is also known by these names:
| Context | Alias |
|---|---|
| plural | UNITS OF MEASUREMENT |
Change to Class: New Class
| UNIT OF MEASUREMENT |
Change to Class: New Class
| may be recorded for one or more CLINICAL INVESTIGATION RESULT ITEM | |
| may be recorded for one or more MALIGNANT ABNORMALITY | |
| may be recorded for one or more PERSON PROPERTY | |
| may be recorded for one or more PRESCRIBED ITEM |
Change to Class: Changed Attributes
| K | BED AVAILABILITY START DATE | |
| AUGMENTED CARE LOCATION CODE | ||
| BED AVAILABILITY END DATE | ||
| IC OR HD BEDS INDICATOR | ||
| WARD AVAILABLE BED |
Change to Class: Changed Attributes
| K | WARD OPERATIONAL PLAN START DATE | |
| AGE GROUP INTENDED | ||
| CLINICAL CARE INTENSITY | ||
| PLANNED WARD LISTING TIME | ||
| SEX OF PATIENTS | ||
| WARD DAY NIGHT INDICATOR | ||
| WARD DAY PERIOD AVAILABILITY | ||
| WARD NIGHT PERIOD AVAILABILITY | ||
| WARD OPERATIONAL PLAN END DATE | ||
| WARD PLAN TOTAL BED CONSULTANT CARE | ||
| WARD PLAN TOTAL BED MIDWIFE ACCESS | ||
| WARD PLAN TOTAL BED NURSING CARE |
Change to Attribute: Changed Description
An ACTIVITY may have many DATES AND TIMES associated with it but may only have one DATE AND TIME of a particular type.
National Codes:
Note: This list is not in alphabetical order.
Change to Attribute: Changed Description
An ACTIVITY may have many TIMES associated with it but may only have one TIME of a particular type.
National Codes:
| 50 | Accident and Emergency Attendance Conclusion Time |
| 51 | Accident and Emergency Departure Time |
| 52 | Accident and Emergency Initial Assessment Time |
| 53 | Accident and Emergency Time Seen For Treatment |
| 54 | Arrival At Hospital Time (Retired April 2012) |
| 55 | ARRIVAL TIME (Retired April 2012) |
| 56 | End Time |
| 57 | Event Time (Retired July 2012) |
| 58 | Initial Patient Contact Time (Retired July 2012) |
| 59 | Last Dosage Time |
| 60 | Pathology Result Due Time |
| 61 | Start Time |
| 62 | Theatre Case Time In To Theatre Suite (Retired September 2012) |
| 63 | Theatre Case Time Out Of Theatre (Retired September 2012) |
| 64 | Theatre Case Time Out Of Theatre Suite (Retired September 2012) |
| 65 | Time Seen |
| 66 | Discharge Ready Time (Retired April 2012) |
| 67 | Arrival Time At Accident and Emergency Department |
| 68 | Arrival Time For Transport Requests |
| 69 | Discharge Time |
| 70 | Clinical Intervention Time |
Note: This list is not in alphabetical order.
Change to Attribute: Changed Description
The type of treatment or care which was delivered in a Cancer Treatment Period.
National Codes:
| 01 | Surgery |
| 02 | Anti-Cancer Drug Regimen (Cytotoxic Chemotherapy) |
| 03 | Anti-Cancer Drug Regimen (Hormone Therapy) |
| 04 | Chemoradiotherapy |
| 05 | Teletherapy (Beam Radiation excluding Proton Therapy) |
| 06 | Brachytherapy |
| 07 | Specialist Palliative Care |
| 08 | Active Monitoring (excluding Non-Specialist Palliative Care) |
| 09 | Non-Specialist Palliative Care (excluding Active Monitoring) |
| 10 | Radiofrequency Ablation (RFA) |
| 11 | High Intensity Focused Ultrasound (HIFU) |
| 12 | Cryotherapy |
| 13 | Proton Therapy |
| 14 | Anti-Cancer Drug Regimen (other) |
| 15 | Anti-Cancer Drug Regimen (Immunotherapy) |
| 16 | Light Therapy (including Photodynamic Therapy and Psoralen and Ultraviolet A Therapy (PUVA)) |
| 17 | Hyperbaric Oxygen Therapy |
| 18 | Other Treatment (Retired 1 July 2012) |
| 19 | Radioisotope Therapy (including Radioiodine) |
| 20 | Laser Treatment (including Argon Beam therapy) |
| 21 | Biological Therapies (excluding Immunotherapy) |
| 22 | Radiosurgery |
| 97 | Other treatment |
| 98 | All treatment declined |
Notes:
- National Code 07 'Specialist Palliative Care', should only be used where care is being delivered under the management of a CONSULTANT in Palliative Medicine.
- National Code 09 'Non-Specialist Palliative Care (excluding Active Monitoring)' is only to be used where the treatment consists of Palliative Care not under the management of a CONSULTANT in Palliative Medicine.
- National Code 09 'Non-Specialist Palliative Care (excluding Active Monitoring)' should only be used to record an ACTIVITY where there is no intention to offer a future course of treatment other than those contained within National Codes 07, 08 or 09 at the time the CARE PLAN is agreed between clinician and PATIENT. This type of care is sometimes referred to as ‘best supportive care’ within NHS services.
Change to Attribute: Changed Description
The type of CLINICAL INTERVENTION.
National Codes:
Change to Attribute: New Attribute
The type of CLINICAL INVESTIGATION RESULT ITEM.
National Codes:
This attribute is also known by these names:
| Context | Alias |
|---|---|
| plural | CLINICAL INVESTIGATION RESULT ITEM TYPES |
Change to Attribute: New Attribute
The recorded value for a CLINICAL INVESTIGATION RESULT ITEM.
A UNIT OF MEASUREMENT may be recorded for a CLINICAL INVESTIGATION RESULT VALUE.
This attribute is also known by these names:
| Context | Alias |
|---|---|
| plural | CLINICAL INVESTIGATION RESULT VALUES |
Change to Attribute: New Attribute
Data Elements:
Change to Attribute: Changed Description
The reason why a delay occurred between the CANCER REFERRAL TO TREATMENT PERIOD START DATE and the DATE FIRST SEEN, when the PRIORITY TYPE of the SERVICE REQUEST was National Code 3 'Two Week Wait'.
This is the reason why the Health Care Provider was unable to provide an APPOINTMENT DATE within the service standard of two weeks.
National Codes:
| 01 | Clinic cancellation |
| 02 | Out-patient capacity inadequate (i.e. no cancelled clinic, but not enough slots for this PATIENT) |
| 03 | Administrative delay |
| 04 | Referral not received within 24 hours (Retired 1 July 2012) |
| 05 | PATIENT unavailable (the PATIENT has declined the opportunity to be seen within two weeks prior to any APPOINTMENT being offered) |
| 06 | PATIENT declines (the PATIENT declines all APPOINTMENT dates offered within two weeks) |
| 07 | PATIENT cancellation (the PATIENT cancels their booked APPOINTMENT) |
| 08 | PATIENT care not commissioned by the English NHS (waiting time standard does not apply) |
| 98 | Other reason |
| 99 | Other reason (Retired 1 July 2012) |
Notes:
- National Code 03 ‘Administrative delay’ should not be used to record delays linked to a ‘Did Not Attend’ (DNA) event where a waiting time adjustment has been entered into the PATIENT record.
- If National Code 98 'Other reason' is used, further detail must be recorded for the precise cause of the delay, within DELAY REASON COMMENT (FIRST SEEN).
- National Code 08 'PATIENT care not commissioned by the English NHS (waiting time standard does not apply)' should only be used in instances where the non-English administration has commissioned a two week wait service, i.e. the PRIORITY TYPE of the SERVICE REQUEST was National Code 03 'Two Week Wait', but the PATIENT was not seen within two weeks. This is to allow for different commissioning arrangements to be supported by local administrative and clinical systems.
Change to Attribute: Changed Description
The reason why a Cancer Care Spell Delay was experienced with regard to a Cancer Care Spell.
The national codes to be used are the same for delays between:
This is the reason why the Health Care Provider was unable to offer a DATE within the service standard (31 days between DECISION TO TREAT DATE and TREATMENT START DATE FOR CANCER, and CONSULTANT UPGRADE DATE and TREATMENT START DATE FOR CANCER; or 62 days between the CANCER REFERRAL TO TREATMENT PERIOD START DATE and TREATMENT START DATE FOR CANCER).
National Codes:
Delays relating to diagnostic and pre-treatment events
| Delays relating to diagnostic and pre-treatment events | |
| 01 | Clinic cancellation |
| 02 | Out-patient capacity inadequate (i.e. no cancelled clinic, but not enough slots for this PATIENT) |
| 03 | Administrative delay |
| 07 | Complex diagnostic pathway (many, or complex, diagnostic tests required) |
| 08 | Delay due to referral between Trusts (Retired 1 July 2012) |
| 11 | Diagnosis delayed for medical reasons (PATIENT unfit for diagnostic episode, excluding planned recovery period following diagnostic test) |
| 13 | Delay due to recovery after an invasive test (PATIENT DIAGNOSIS or treatment delayed due to planned recovery period following an invasive diagnostic test) |
| 17 | PATIENT choice delay relating to first outpatient APPOINTMENT |
| 18 | Health Care Provider initiated delay to diagnostic test or treatment planning |
| 19 | PATIENT initiated (choice) delay to diagnostic test or treatment planning, advance notice given |
| 20 | PATIENT Did Not Attend an APPOINTMENT for a diagnostic test or treatment planning event (no advance notice) |
| 98 | Other reason |
| Delays relating to treatment in an admitted care setting | |
| 04 | Elective cancellation (for non-medical reason) |
| 05 | Elective capacity inadequate (PATIENT unable to be scheduled for treatment within standard time) |
| 06 | Delay to diagnostic test or treatment planning (Retired 1 July 2012) |
| 10 | Treatment delayed for medical reasons (PATIENT unfit for treatment episode, excluding planned recovery period following diagnostic test) |
| 21 | PATIENT failed to present for elective treatment (choice) |
| 22 | PATIENT care not commissioned by the English NHS (waiting time standard does not apply) |
| 98 | Other reason |
| Delays relating to treatment in a non-admitted care setting | |
| 01 | Clinic cancellation |
| 02 | Out-patient capacity inadequate (i.e. no cancelled clinic, but not enough slots for this PATIENT) |
| 10 | Treatment delayed for medical reasons (PATIENT unfit for treatment episode, excluding planned recovery period following diagnostic test) |
| 14 | PATIENT Did Not Attend treatment APPOINTMENT |
| 16 | PATIENT Choice (PATIENT declined or cancelled an offered APPOINTMENT DATE for treatment) |
| 22 | PATIENT care not commissioned by the English NHS (waiting time standard does not apply) |
| 98 | Other reason |
| 99 | Other reason (Retired 1 July 2012) |
Notes:
- If National Code 98 'Other reason' is used, the reason must be explained within DELAY REASON COMMENT (CONSULTANT UPGRADE), DELAY REASON COMMENT (REFERRAL TO TREATMENT) or DELAY REASON COMMENT (DECISION TO TREATMENT) as appropriate.
- National Code 03 'Administrative delay' should not be used to record delays linked to a ‘Did Not Attend’ (DNA) event where a waiting time adjustment has been entered into the PATIENT record.
- National Codes 04, 05 and 21 can only be used where the treatment was delivered in an admitted care setting i.e. where the CANCER CARE SETTING (TREATMENT) is National Code 01 or 02.
- National Codes 14 and 16 can only be used where the treatment was delivered in a non-admitted care setting i.e. where the CANCER CARE SETTING (TREATMENT) is National Code 03 or 04.
- National Code 17 should only be used where DELAY REASON REFERRAL TO FIRST SEEN (CANCER OR BREAST SYMPTOMS) is also present in the PATIENT record.
- National Code 20 should not be used for any Did Not Attend (DNA) event relating to DATE FIRST SEEN. Events of this type should not constitute a delay as they can be accounted for by entering a value for WAITING TIME ADJUSTMENT (FIRST SEEN) in the PATIENT record.
- National Codes 07, 11, 13,17,18,19 and 20 should only be used for Referral to Treatment type pathways, therefore these should not be used to record a value for DELAY REASON COMMENT (DECISION TO TREATMENT).
- National Code 22 'PATIENT care not commissioned by the English NHS (waiting time standard does not apply)' should only be used in instances where the non-English administration has commissioned a cancer service with similar ‘target times’ and data item attributes. This is to allow different commissioning arrangements to be supported by a single local administrative and clinical system.
- If a delay to the pathway is due to an administrative delay in the transfer of a PATIENT from one Health Care Provider to another (an Inter-Provider Transfer or IPT) this should be recorded as National Code 03 'Administrative delay' with appropriate supporting detail given in either DELAY REASON COMMENT (REFERRAL TO TREATMENT) or DELAY REASON COMMENT (CONSULTANT UPGRADE).
Change to Attribute: Changed Description
Change to Attribute: New Attribute
The gestation length of a Fetus Episode recorded as the total number of days.
The calculation may be:
| a) | calculated by ultrasound scan measurements according to the trimester of the scan |
| b) | estimated from the LAST MENSTRUAL PERIOD DATE |
| c) | estimated by clinical assessment (in the absence of a or b) |
The number of completed whole weeks of gestation and the remaining number of days of an uncompleted whole week should be calculated from the GESTATION LENGTH IN DAYS for input and reporting purposes. Where there is no uncompleted whole week, the number of additional days should be recorded as zero.
For example:
This attribute is also known by these names:
| Context | Alias |
|---|---|
| plural | GESTATION LENGTHS IN DAYS |
Change to Attribute: New Attribute
Change to Attribute: Changed Name
- Changed Name from Data_Dictionary.Attributes.G.GESTATION_LENGTH to Data_Dictionary.Attributes.G.GESTATION_LENGTH_IN_WEEKS
Change to Attribute: Changed Description
Where the neck has been dissected during a Head and Neck Cancer Care Spell, the size of the largest metastasis, in 'Millimetres (mm)'.Where the neck has been dissected during a Head and Neck Cancer Care Spell, the size of the largest metastasis, where the UNIT OF MEASUREMENT is 'Millimetres'.
Change to Attribute: Changed Description
The maximum depth of invasion of the Tumour, in 'Millimetres (mm)'.The maximum depth of invasion of the Tumour, where the UNIT OF MEASUREMENT is 'Millimetres' .
Change to Attribute: Changed Name, Description, status to Retired
The recorded value for the MEASURED PERSON OBSERVATION observed associated with a MEASURED PERSON OBSERVATION TYPE CODE and MEASUREMENT VALUE TYPE CODE.This item has been retired from the NHS Data Model and Dictionary.
Access to this version can be obtained by emailing information.standards@hscic.gov.uk with "NHS Data Model and Dictionary - Archive Request" in the email subject line.
Change to Attribute: Changed Name, Description, status to Retired
- Changed Name from Data_Dictionary.Attributes.M.MEASURED_OBSERVATION_VALUE to Retired.Data_Dictionary.Attributes.M.MEASURED_OBSERVATION_VALUE
- Changed Description
- Retired MEASURED OBSERVATION VALUE
Change to Attribute: Changed Name, Description, status to Retired
The type of MEASURED PERSON OBSERVATION.This item has been retired from the NHS Data Model and Dictionary.
Each MEASURED PERSON OBSERVATION TYPE CODE must have an associated MEASUREMENT VALUE TYPE.The last live version of this item is available in the September 2013 release of the NHS Data Model and Dictionary.
National Codes:Access to this version can be obtained by emailing information.standards@hscic.gov.uk with "NHS Data Model and Dictionary - Archive Request" in the email subject line.
Change to Attribute: Changed Name, Description, status to Retired
- Changed Name from Data_Dictionary.Attributes.M.MEASURED_PERSON_OBSERVATION_TYPE_CODE to Retired.Data_Dictionary.Attributes.M.MEASURED_PERSON_OBSERVATION_TYPE_CODE
- Changed Description
- Retired MEASURED PERSON OBSERVATION TYPE CODE
Change to Attribute: Changed Name, Description, status to Retired
The type of measurement used for the MEASURED PERSON OBSERVATION being recorded.This item has been retired from the NHS Data Model and Dictionary.
National Codes:The last live version of this item is available in the September 2013 release of the NHS Data Model and Dictionary.
Change to Attribute: Changed Name, Description, status to Retired
- Changed Name from Data_Dictionary.Attributes.M.MEASUREMENT_VALUE_TYPE_CODE to Retired.Data_Dictionary.Attributes.M.MEASUREMENT_VALUE_TYPE_CODE
- Changed Description
- Retired MEASUREMENT VALUE TYPE CODE
Change to Attribute: Changed Description
An ORGANISATION DEPARTMENT CODE is a code which identifies an ORGANISATION DEPARTMENT uniquely.
For NHS ORGANISATIONS it is a code that is managed by either the:
Notes:
Organisation Data Servicecodes can be downloaded:via files issued by theTechnology Reference Data Update Distribution Service (TRUD)
- Organisation Data Service codes can be downloaded:
- via files issued by the Technology Reference Data Update Distribution Service (TRUD)
- Organisation Data Service contact details can be found at Contact Details.
ORGANISATION DEPARTMENT CODING FRAMES
- All NHS ORGANISATION DEPARTMENTS are coded using coding frames, as shown in the tables below:
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
Format | a/n | a/n | a/n | a/n | a/n | a/n | a/n | a/n |
A Frame | Department Type Identifier | Department Identifier | ||||||
B Frame | Department Type Identifier | Department Identifier | ||||||
C Frame | Department Type Identifier | Department Identifier | ||||||
A Frame:
Example
Pathology Laboratory e.g. 69010
- 69 = Department Type Identifier
- 010 = Department Identifier
B Frame:
Example
Local Authority Department e.g. V002AC01
- V = Department Type Identifier
- 002AC01 = Department Identifier
C Frame:
Example
Isle of Man Government Department e.g. YJ1
- YJ = Department Type Identifier
- 1 = Department Identifier
The structure and format of ORGANISATION DEPARTMENT CODES maintained by the Organisation Data Service, NHS Prescription Services and other agencies are detailed in the tables below.
ORGANISATION CODES TABLES
Table 1: CODING FORMATS FOR ORGANISATION DEPARTMENTS IN ENGLAND AND WALES
Organisation Department Type | Frame Type | Character Position | Code allocated by: | Notes/Comments | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||||
Executive Agency Programme Department | N/A | X | 0-9 | 0-9 | 0-9 | 0-9 | 0-9 | A-Y | A-Y | First six characters denote Executive Agency Programme e.g. X09001AA | |
Local Authority Department | B | V | A-9 | A-9 | A-9 | A-Y | A-Y | A-9 | A-9 | e.g. V002AC01 | |
A | 6 | 9 | A-9 | A-9 | A-9 | e.g. 69010 | |||||
Note: Codes for Executive Agency, Executive Agency Programme, Executive Agency Site and Executive Agency Programme Department do not easily fit into the coding frames as shown above and are therefore not included. This is due to their unusual structure in that there are more hierarchical 'tiers' than with other organisations.
Executive Agency and Executive Agency Programme are both considered Organisation level entities, although each Programme does have a relationship to an Executive Agency. Executive Agency codes are three characters long. Executive Agency Programme codes are six, and their first three characters are the same as the Executive Agency they are associated to.
Department codes of eight characters long can then be allocated underneath a Programme code (sharing the first six characters). Executive Agency Site codes of five characters long can be allocated under an Executive Agency code (and share the first three characters).
Table 2: CODING FORMATS FOR ORGANISATION SITES IN OTHER HOME COUNTRIES
Organisation Department Type | Frame Type | Character Position | Code allocated by: | Notes/Comments | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||||
Isle of Man Government Department | C | Y | J | A-9 |
| e.g. YJ1 | |||||
Note: A-9 indicates that characters A-Z and 0-9 are valid: except B, I, O, S, U and Z (to avoid ambiguity).
Change to Attribute: Changed Description
An ORGANISATION SITE CODE is a code which identifies an ORGANISATION SITE uniquely.
Note: Only ORGANISATION SITE CODES which have been notified to and issued by the Organisation Data Service may be used.
Notes:
Organisation Data Servicecodes can be downloaded:from theOrganisation Data Service websiteandvia files issued by theTechnology Reference Data Update Distribution Service (TRUD)
- Organisation Data Service codes can be downloaded:
- via files issued by the Technology Reference Data Update Distribution Service (TRUD)
- Organisation Data Service contact details can be found at Contact Details.
ORGANISATION SITE CODING FRAMES
- All NHS ORGANISATION SITES are coded using coding frames, as shown in the tables below:
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
Format | a/n | a/n | a/n | a/n | a/n | a/n | a/n | a/n | a/n |
A Frame | Organisation Type Identifier | Organisation Identifier | Site or Sub-Division Identifier | ||||||
B Frame | Organisation Type Identifier | Organisation Identifier | Site or Sub-Division Identifier | ||||||
C Frame | Organisation Type Identifier | Organisation Identifier | Site or Sub-Division Identifier | ||||||
D Frame | Organisation Type | Practice Identifier | Branch Surgery Identifier | ||||||
E Frame | Organisation Type Identifier | Organisation Identifier | Site or Sub-Division Identifier | ||||||
F Frame | Organisation Type | Organisation Identifier | |||||||
H Frame | Organisation Type Identifier | Organisation Identifier | |||||||
I Frame | Organisation Type Identifier | Organisation Identifier | |||||||
J Frame | Organisation Type Identifier | Organisation Identifier | |||||||
K Frame | Organisation and Organisation Type Identifier | Organisation Site Identifier | |||||||
A Frame:
Example
Local Service Provider Site e.g. LSP0101
- LSP = Org Type Identifier
- 01 = Organisation Identifier
- 01 = Site or Sub-Division Identifier
B Frame:
Example
Care Trust Site e.g. TAK01
- T = Organisation Type Identifier
- AK = Organisation Identifier
- 01 = Site or Sub-Division Identifier
Also:
| Government Department Site | e.g. XDA01 |
| High Level Health Geography Site, e.g. Area Team site | e.g. Q4401 |
| Local Authority Site | e.g. 000AA |
| Local Health Board (Wales) Site | e.g. 7A101 |
| NHS Trust Site | e.g. RH802 |
| Other Statutory Authority (OSA) Site | e.g. X1601 |
| e.g. Q3001 |
C Frame:
Example
Independent Sector Healthcare Provider (ISHP) Site e.g. NV701
- NV = Organisation Site Type Identifier
- 7 = Organisation Identifier
- 01 = Site or Sub-Division Identifier
D Frame
Example
GP Practice Branch Surgery: e.g. H81010002
- H (and length of code) = Organisation Identifier
- 81010 = Organisation Identifier (parent GP Practice)
- 002 = Branch Surgery Identifier
E Frame
Example
Special Health Authority (SpHA) Site: e.g. T115A
- T1 = Organisation Type Identifier
- 15 = Organisation Identifier
- A = Site or Sub-Division Identifier
F Frame
Example
Commissioning Support Unit Site: e.g. 0AA01
- 0 = Organisation Type Identifier
- AA01 = Organisation Identifier
H Frame
Example
Prison: e.g. YDE01
- YDE = Organisation Type Identifier
- 01 = Site or Sub-Division Identifier
I Frame
Example
Optical Site: e.g. TP01A
- TP = Organisation Type Identifier
- 01A = Site or Sub-Division Identifier
J Frame
Example
Care Home Site: e.g. VN01A
- VN = Organisation Type Identifier
- 01A = Site or Sub-Division Identifier
Also:
| Health Observatory | e.g. XP001 |
| Primary Healthcare Directorate (Isle of Man) Site | e.g. YK101 |
K Frame
Example
Clinical Commissioning Group (CCG) Site e.g. 11AAA - 99YZZ
- 11A = Organisation and Organisation Type Identifier
- AA = Organisation Site Identifier
The structure and format of ORGANISATION SITE CODES maintained by the Organisation Data Service, NHS Prescription Services and other agencies are detailed in the tables below.
NHS ORGANISATION SITE CODES TABLES
Coding Formats
Table 1: CODING FORMATS FOR ORGANISATION SITES IN ENGLAND AND WALES
Organisation Site Type | Frame Type | Character Position | Code allocated by: | Notes/Comments | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||||
Care Home Site | J | V | L, M or N | A-9 | A-9 | A-9 | e.g. VN01A, VM01A, VL01A | |||||
Care Trust Site | B | T | A-Y | A-Y | A-9 | A-9 | First three characters denote owning Care Trust e.g. TAK01 | |||||
| Clinical Commissioning Group (CCG) Site | K | 0-9 | 0-9 | A-Y | A-Y | A-Y | First three characters denote owning Clinical Commissioning Group e.g. 11AAA - 99YZZ | |||||
Commissioning Support Unit (CSU) Site | F | 0 | A-Y | A-Y | A-9 | A-9 | e.g. 0AA01 | |||||
Executive Agency Site | N/A | X | 0-9 | 0-9 | 0-9 | 0-9 | First three characters denote Executive Agency e.g. X0901 | |||||
Government Department Site | B | X | A-Y | A-Y | 0-9 | 0-9 | First three characters denote Government Department e.g. XDA01 | |||||
GP Practice Branch Surgery - England and Wales | D | A-H, | 0-9 | 0-9 | 0-9 | 0-9 | 0-9 | 0-9 | 0-9 | 0-9 | First 6 characters denote parent practice. Char 1 = W for Welsh GP Practice. All other values represent English GP Practices e.g. H81010002 | |
| Health Observatory | J | X | P | 0-9 | 0-9 | 0-9 | e.g. XP001 | |||||
High Level Health Geography Site, e.g. Area Team site
| B | Q | A-9 | A-9 | A-9 | A-9 | e.g. Q4401 | |||||
C | A, B, D, G, I, K, L, M , N, O, S, U, V, W | A-Y | A-Y, 0-9 | A-Y, 0-9 | A-Y, 0-9 | First three characters denote owning Independent Sector Healthcare Provider (ISHP) e.g. NV701 Note: The A-Y range includes all letters except Z | ||||||
Local Authority (LA) Site | B | 0-9 | 0-9 | 0-9 | A-Z | A-Z | First three characters denote parent Local Authority e.g. 000AA | |||||
| Local Health Board (Wales) Site | B | 7 | A-9 | A-9 | A-9 | A-9 | First three characters denote owning NHS Trust e.g. 7A101 | |||||
Local Service Provider Site | A | L | S | P | 0-9 | 0-9 | 0-9 | 0-9 | First five characters denote owning Local Service Provider e.g. LSP0101 | |||
NHS Trust Site | B | R | A-9 | A-9 | A-9 | A-9 | First three characters denote owning NHS Trust e.g. RH802 | |||||
I | T | P or Q | 0-9 | A-9 | A-9 | e.g. TP01A, TQ01A | ||||||
Other Statutory Authority (OSA) Site | B | X | 0-9 | 0-9 | 0-9 | 0-9 | First three characters denote owning Other Statutory Authority e.g. X1601 | |||||
Primary Care Trust (PCT) Site | B | 5 | A-9 | A-9 | A-9 | A-9 | First three characters denote owning Primary Care Trust e.g. 5CT49 All Primary Care Trusts closed 31 March 2013 | |||||
Special Health Authority (SpHA) Site | E | T | 1 | 0-9 | 0-9 | A-Y, 1-9 | First three characters denote owning SpHA e.g. T115A | |||||
Strategic Health Authority (SHA) Site | B | Q | A-9 | A-9 | A-9 | A-9 | First three characters denote owning SHA Trust e.g. Q3001 All Strategic Health Authorities closed 31 March 2013 - from 1 April 2013 referred to High Level Health Geography Site | |||||
Note: Codes for Executive Agency, Executive Agency Programme, Executive Agency Site and Executive Agency Programme Department do not easily fit into the coding frames as shown above and are therefore not included. This is due to their unusual structure in that there are more hierarchical 'tiers' than with other organisations.
Executive Agency and Executive Agency Programme are both considered Organisation level entities, although each Programme does have a relationship to an Executive Agency. Executive Agency codes are three characters long. Executive Agency Programme codes are six, and their first three characters are the same as the Executive Agency they are associated to.
Department codes of eight characters long can then be allocated underneath a Programme code (sharing the first six characters). Executive Agency Site codes of five characters long can be allocated under an Executive Agency code (and share the first three characters).
Note: A-9 indicates that characters A-Z and 0-9 are valid: except B, I, O, S, U and Z (to avoid ambiguity). This applies to all ORGANISATION SITE CODES in the Coding Format Table above except Independent Sector Healthcare Provider (ISHP) sites.
Table 2: CODING FORMATS FOR ORGANISATION SITES IN OTHER HOME COUNTRIES
Organisation Site Type | Frame Type | Character Position | Code allocated by: | Notes/Comments | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||||
J | Y | K | A-9 | A-9 | A-9 |
| e.g. YK101 | |||||
Note: A-9 indicates that characters A-Z and 0-9 are valid: except B, I, O, S, U and Z (to avoid ambiguity).
Change to Attribute: Changed Name, Description, status to Retired
Identifies the type of qualifier intrinsic to the PERSON PROPERTY.This item has been retired from the NHS Data Model and Dictionary.
Access to this version can be obtained by emailing information.standards@hscic.gov.uk with "NHS Data Model and Dictionary - Archive Request" in the email subject line.
Change to Attribute: Changed Name, Description, status to Retired
- Changed Name from Data_Dictionary.Attributes.P.Person.PERSON_PROPERTY_QUALIFIER_TYPE to Retired.Data_Dictionary.Attributes.P.PERSON_PROPERTY_QUALIFIER_TYPE
- Changed Description
- Retired PERSON PROPERTY QUALIFIER TYPE
Change to Attribute: Changed Name, Description, status to Retired
The value of the qualifier intrinsic to the PERSON PROPERTY. The appropriate value is determined by the PERSON PROPERTY QUALIFIER TYPE.This item has been retired from the NHS Data Model and Dictionary.
Access to this version can be obtained by emailing information.standards@hscic.gov.uk with "NHS Data Model and Dictionary - Archive Request" in the email subject line.
Change to Attribute: Changed Name, Description, status to Retired
- Changed Name from Data_Dictionary.Attributes.P.Person.PERSON_PROPERTY_QUALIFIER_VALUE to Retired.Data_Dictionary.Attributes.P.PERSON_PROPERTY_QUALIFIER_VALUE
- Changed Description
- Retired PERSON PROPERTY QUALIFIER VALUE
Change to Attribute: Changed Name, Description, status to Retired
Identifies the type of association from one PERSON PROPERTY to another.This item has been retired from the NHS Data Model and Dictionary.
Access to this version can be obtained by emailing information.standards@hscic.gov.uk with "NHS Data Model and Dictionary - Archive Request" in the email subject line.
Change to Attribute: Changed Name, Description, status to Retired
- Changed Name from Data_Dictionary.Attributes.P.Person.PERSON_PROPERTY_RELATIONSHIP_TYPE to Retired.Data_Dictionary.Attributes.P.PERSON_PROPERTY_RELATIONSHIP_TYPE
- Changed Description
- Retired PERSON PROPERTY RELATIONSHIP TYPE
Change to Attribute: Changed Description
National Codes:
| 1 | Specialised Rehabilitation Team |
| 2 | Non-specialised Rehabilitation Team |
Note:This data item is included in Commissioning Data Set version 6-2, but should not be submitted until further development by the Department of Health has been undertaken.This data item is included in Commissioning Data Set version 6-2, but should not be submitted until further development by the Department of Health has been undertaken.
Change to Attribute: Changed Description
The type of SERVICE.
National Codes:
Change to Attribute: Changed Description
The type of SERVICE providing chlamydia testing.
National Codes:
| 01 | Genitourinary Medicine Services | Includes testing done in Genitourinary Medicine clinics reported to Genitourinary Medicine Clinic Activity Data Set (GUMCAD). |
| 02 | Community Sexual Health Services | Includes testing carried out in Sexual and Reproductive Health Services/Contraception and Sexual Health (CASH) services/Community Contraceptive Services excludes Contraceptive Services within GP Practices. Includes young PERSON's sexual health services e.g. Brook clinics and SexSense. It also includes pre-instrumentation screening e.g. Intrauterine Devices where undertaken at CASH services and postal kits handed out at community sexual health services. |
| 03 | GP Practice | Includes post kits handed out at the GP Practice. |
| 04 | Pharmacy | Includes testing carried out in community pharmacies, including post kits handed out at the pharmacy. |
| 05 | Termination of Pregnancy (TOP) Services | Includes testing undertaken in TOP services at all stages including medical and surgical. Includes all NHS and private providers including British Pregnancy Advice Service (BPAS), Marie Stopes and Pregnancy Crisis Centre. It also includes post kits handed out in TOP Services. |
| XX | Other | Any other testing service type which does not fit into categories 01 - 05 e.g. chlamydia screening offices, antenatal and obstetric services, military,education, occupational health, prison, youth services, outreach, accident and emergency, minor injuries, NHS walk-in centres and Hospitals. |
Change to Attribute: Changed Description
The SKIN CANCER LESION identification number used to identify the specimen within a Pathology Laboratory Service Report during a Skin Cancer Care Spell.The identification number used to identify the specimen within a Pathology Laboratory Service Report during a Skin Cancer Care Spell.
Change to Attribute: Changed Description
National Codes:
| 1 | Less than or equal to 20mm |
| 2 | Greater than 20mm |
Change to Attribute: Changed Description
Change to Attribute: Changed Name, Description
The identification of the unit of measurement for a CLINICAL INVESTIGATION RESULT ITEM.The unit of measurement.
National Codes:
| 01 | Millimoles per litre (mmol/L) |
| 02 | Micromoles per litre (µmol/L) |
| 03 | Micrograms per litre (µg/L) |
| 04 | Micrograms per millimole (µg/mmol) |
| 05 | Microgram albumin per hour (µg/ml/hr) |
| 06 | Microgram albumin per minute (µg/min) |
| 07 | Microgram albumin per 24 hours (µg/24hr) |
| 08 | Number (Retired September 2013) |
| 09 | Percentage (%) |
| 10 | Kilograms (kg) |
| 11 | Metres (m) |
| 12 | Picograms (pg) |
| 13 | Square Metres (m2) |
| 14 | Millilitres per Minute (ml/min) |
| 15 | Millimetres of mercury (mmHg) |
| 16 | Litres (l) |
| 17 | Beats per minute (bpm) |
| 18 | Centimetres (cm) |
| 19 | Milligrams (mg) |
| 20 | Millilitres (ml) |
| 21 | Minutes |
| 22 | Celsius (ºC) |
| 23 | Millimetres (mm) |
| 24 | Grams per decilitre (g/dl) |
| 25 | Grams per litre (g/l) |
| 26 | Milligrams per litre (mg/l) |
| 27 | Nanograms per millilitre (ng/ml) |
| 28 | International Units per litre (IU/L) |
| 29 | Decilitres (d/l) |
| 30 | Square Millimetres (mm2) |
| 31 | Millilitres (ml) (Retired September 2013) |
| 32 | Grays (Gy) |
| 33 | International Units per kilogram (IU/kg) |
| 34 | Grams (g) |
| 35 | Kilocalories (kcal) |
| 36 | Millimoles (mmol) |
| 37 | Millimoles per mole (mmol/mol) |
| 38 | Picomoles per litre (pmol/L) |
| 39 | Milligrams per millimole (mg/mmol) |
| 40 | Nanograms per litre (ng/l) |
| 41 | Micrograms per millilitre (µg/ml) |
| 42 | Millimetres of water (mmH2O) |
| 43 | Cubic Millimetres (mm3) |
| 44 | Litres per week per 1.73 metres squared (l/week/1.73²) |
| 45 | Millilitres per Minute divided by 1.73 Square Metres (ml/min/1.73m2) |
| 46 | number times ten raised to the power of nine per litre (x109/l) |
| 47 | 5 Millimetres Squared |
| 48 | Grams per kilogram per day (g/kg/day) |
| 49 | Kilopascals (KPa) |
| 50 | Femtolitres (fl) |
| 51 | Megavolts |
References:
The Version 1.1 NHS Standard EDIFACT Messages for Pathology Requests and Reports, 2001
The Version 1.0 Trial NHS Standard EDIFACT Messages for GP-Hospital Communications - 17.5.95
Change to Attribute: Changed Name, Description
- Changed Name from Data_Dictionary.Attributes.C.Cla.CLINICAL_INVESTIGATION_RESULT_ITEM_UNIT_OF_MEASURE to Data_Dictionary.Attributes.U.UNIT_OF_MEASUREMENT
- Changed Description
Change to Data Element: Changed Description
| Format/Length: | an1 |
| HES Item: | |
| National Codes: | See ABLATIVE THERAPY TYPE |
| Default Codes: | 9 - Not Known (Not Recorded) |
Notes:
ABLATIVE THERAPY TYPE is the same as attribute ABLATIVE THERAPY TYPE.
Change to Data Element: Changed Description
| Format/Length: | an1 |
| HES Item: | |
| National Codes: | See ABNORMALITY DETECTED INDICATOR |
| Default Codes: |
Notes:
ABNORMALITY DETECTED (DATING ULTRASOUND SCAN) is the same as the attribute ABNORMALITY DETECTED INDICATOR for a Dating Ultrasound Scan.
Change to Data Element: Changed Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
ACCIDENT AND EMERGENCY ATTENDANCE CONCLUSION DATE is the same as attribute ACTIVITY DATE where the ACTIVITY DATE TYPE is National Code 'Accident and Emergency Attendance Conclusion Date'.
Change to Data Element: Changed Description
| Format/Length: | an6 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
See Accident and Emergency Diagnosis Tables for clinical coding and classification structure.
Accident and Emergency Attendance is a CARE CONTACT where the CARE CONTACT TYPE is National Code 01 'Accident And Emergency Attendance'.For Commissioning Data Set and XML schema version 6 onwards, ACCIDENT AND EMERGENCY DIAGNOSIS - FIRST will be recognised as Primary Diagnosis (Accident and Emergency)
For Commissioning Data Set and XML schema version 6, this Data Element will be recognised as Primary Diagnosis (Accident and Emergency)
Change to Data Element: Changed Description
| Format/Length: | an6 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
See Accident and Emergency Diagnosis Tables for clinical coding and classification structure.
Accident and Emergency Attendance is a CARE CONTACT where the CARE CONTACT TYPE is National Code 01 'Accident And Emergency Attendance'.For Commissioning Data Set and XML Schema version 6 onwards, ACCIDENT AND EMERGENCY DIAGNOSIS - SECOND will be recognised as Secondary Diagnosis (Accident and Emergency).For Commissioning Data Set and XML Schema version 6, this Data Element will be recognised as Secondary Diagnosis (Accident and Emergency).
For Commissioning Data Set version 6 onwards, there are no restrictions on the number of Secondary Diagnoses (Accident and Emergency) recorded.
Change to Data Element: Changed Description
| Format/Length: | an6 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
See Accident and Emergency Investigation Table for clinical coding and classification structure.
Accident and Emergency Attendance is a CARE CONTACT where the CARE CONTACT TYPE is National Code 01 'Accident And Emergency Attendance'.For Commissioning Data Set and XML Schema version 6 onwards, ACCIDENT AND EMERGENCY INVESTIGATION - FIRST will be recognised as Primary Investigation (Accident and Emergency).
For Commissioning Data Set and XML Schema version 6, this Data Element will be recognised as Primary Investigation (Accident and Emergency).
Change to Data Element: Changed Description
| Format/Length: | an6 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
See Accident and Emergency Investigation Table for clinical coding and classification structure.
Accident and Emergency Attendance is a CARE CONTACT where the CARE CONTACT TYPE is National Code 01 'Accident And Emergency Attendance'.
For Commissioning Data Set and Schema version 6 onwards, ACCIDENT AND EMERGENCY INVESTIGATION - SECOND will be recognised as Secondary Investigation (Accident and Emergency).For Commissioning Data Set and Schema version 6, this Data Element will be recognised as Secondary Investigation (Accident and Emergency).
For Commissioning Data Set version 6 onwards there are no restrictions on the number of Secondary Investigations (Accident and Emergency) recorded.
Change to Data Element: Changed Description
| Format/Length: | an6 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
See Accident and Emergency Treatment Tables for clinical coding and classification structure.
Accident and Emergency Attendance is a CARE CONTACT where the CARE CONTACT TYPE is National Code 01 'Accident And Emergency Attendance'.For Commissioning Data Set and XML Schema version 6 onwards, ACCIDENT AND EMERGENCY TREATMENT - FIRST will be recognised as Primary Treatment (Accident and Emergency).
For Commissioning Data Set and XML Schema version 6 this Data Element will be recognised as Primary Treatment (Accident and Emergency).
Change to Data Element: Changed Description
| Format/Length: | an6 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
See Accident and Emergency Treatment Tables for clinical coding and classification structure.
Accident and Emergency Attendance is a CARE CONTACT where the CARE CONTACT TYPE is National Code 01 'Accident And Emergency Attendance'.
For Commissioning Data Set and XML Schema version 6 this Data Element will be recognised as Secondary Treatment (Accident and Emergency).For Commissioning Data Set and XML Schema version 6 onwards, ACCIDENT AND EMERGENCY TREATMENT - SECOND will be recognised as Secondary Treatment (Accident and Emergency).
For Commissioning Data Set version 6 onwards there are no restrictions on the number of Secondary Treatment (Accident and Emergency) recorded.
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
The value is presented in the range 10-80.
For the Cancer Outcomes and Services Data Set, ALBUMIN LEVEL is measured pre-treatment.
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed Description
| Format/Length: | max an200 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
ALLELE DRB3 DONOR is the ORGAN OR TISSUE DONOR's 'DRB3 alleles', as contained in the Human Leukocyte Antigen report for Tissue Typing.
Change to Data Element: Changed Description
| Format/Length: | max an200 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
ALLELE DRB4 DONOR is the ORGAN OR TISSUE DONOR's 'DRB4 alleles', as contained in the Human Leukocyte Antigen report for Tissue Typing.
Change to Data Element: Changed Description
| Format/Length: | max an200 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
ALLELE DRB5 DONOR is the ORGAN OR TISSUE DONOR's 'DRB5 alleles', as contained in the Human Leukocyte Antigen report for Tissue Typing.
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n6 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | n1.max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
ANTENATAL OBSERVATION (MATERNAL HEIGHT) is the Height of the mother measured during an Antenatal period.
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3.max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
ANTENATAL OBSERVATION (MATERNAL WEIGHT) is the Weight of the mother measured during an Antenatal period.
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.max n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
For the Cancer Outcomes and Services Data Set, BETA2 MICROGLOBULIN LEVEL is measured pre-treatment.
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
BETA HUMAN CHORIONIC GONADOTROPIN may also be produced by some Tumour CELLS. An increased level of beta-human chorionic gonadotropin may be a sign of cancer of the testis, uterus, ovary, liver, stomach, pancreas, or lungs.
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | n4 |
| HES Item: | BIRWEIT |
| National Codes: | |
| Default Codes: | 9999 - Not known |
Notes:
The range is 0001 to 9998.
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3/max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3/max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3/max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | n3/n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3.max n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.max n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.max n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | yy.mm |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
For the National Renal Data Set, BONE AGE (RENAL PAEDIATRIC) is the radiological Bone Age as assessed by a radiologist viewing X-rays of the PATIENT's hand and wrist. The age is reported in years and months.
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
The value is presented in the range 0-20%.
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3.max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
For the National Renal Data Set, CALCULATED CREATININE CLEARANCE is for PATIENTS under 18 years only.
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n4 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
If the PATIENT's CD4 cell count has not been recorded, the field should be omitted.
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3.max n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n1.max n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.max n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed Description
| Format/Length: | See COUNTRY CODE |
| NWDS ID: | PNAT |
| NWDS Field Name: | Nationality |
| National Codes: | |
| Default Codes: | 97 - Not recorded |
| 99 - Not known |
Notes:
COUNTRY CODE (AT ASSIGNMENT) is the same as attribute COUNTRY CODE.
COUNTRY CODE (AT ASSIGNMENT) is the nationality of the EMPLOYEE:
- as declared by the individual on appointment for an ASSIGNMENT to a POSITION or
- as advised by the individual in the course of employment (should they change their nationality).
COUNTRY CODE (AT ASSIGNMENT) is the COUNTRY CODE of the COUNTRY where the NATIONALITY INDICATOR of NATIONALITY OR RESIDENCY is National Code 'National of the respective country at birth and still a national' or 'National of respective country subsequent to birth and still a national'.COUNTRY CODE (AT ASSIGNMENT) is the COUNTRY CODE where the NATIONALITY INDICATOR is National Code 'National of the respective country at birth and still a national' or 'National of respective country subsequent to birth and still a national'.
For Electronic Staff Record and National Workforce Data Set usage only one nationality can be identified so in the case of dual nationality, the EMPLOYEE should choose the preferred COUNTRY for recording their nationality.
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.max n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed Description
| Format/Length: | max n4 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
DIALYSATE CREATININE PLASMA RATIO (4 HOUR) is the result of the Clinical Investigation which calculates the ratio of creatinine in dialysate to the creatinine in plasma measured as per The Renal Association guidelines.
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n6 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.max n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.max n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute
Change to Data Element: Changed linked Attribute
Change to Data Element: Changed Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Note: if there is doubt about the sites of the muscularis propria, the distance should be estimated as accurately as possible.
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed Name, Description, status to Retired
This item has been retired from the NHS Data Model and Dictionary.
The last live version of this item is available in the September 2013 release of the NHS Data Model and Dictionary.
Access to this version can be obtained by emailing information.standards@hscic. uk with "NHS Data Model and Dictionary - Archive Request" in the email subject line.
Change to Data Element: Changed Name, Description, status to Retired
- Changed Name from Data_Dictionary.Data_Field_Notes.E.En.ENDOCRINE_THERAPY_TYPE to Retired.Data_Dictionary.Data_Field_Notes.E.ENDOCRINE_THERAPY_TYPE
- Changed Description
- Retired ENDOCRINE THERAPY TYPE
Change to Data Element: Changed Description
| Format/length: | n2 |
| HES item: | EPIORDER |
| National Codes: | |
| Default Codes: | 98 - Not applicable |
| 99 - Not known: a validation error |
Notes:
EPISODE NUMBER is the same as attribute ACTIVITY IDENTIFIER and is used to uniquely identify episodes, and is a sequence number for each Consultant Episode (Hospital Provider) in a Hospital Provider Spell. The first episode of each new Hospital Provider Spell (including re-admitted PATIENTS) commences at 01.
A known EPISODE NUMBER can be between 01 to 87.
For other Health Care Provider episodes, it is a sequence number for a CONSULTANT/PATIENT combination; or it is a sequence number for each Sexual Health And HIV Episode; or it is a sequence number for each Community Episode in a Nursing In The Community Programme.
Each of the above Consultant Episode (Hospital Provider), Hospital Provider Spell, Sexual Health And HIV Episode and Community Episode is an ACTIVITY GROUP where the ACTIVITY GROUP TYPE identifies the specific spell, episode or stay type.
Nursing In The Community Programme is a HEALTH PROGRAMME where HEALTH PROGRAMME TYPE is National Code 03 'Nursing In The Community Programme'.
Change to Data Element: Changed Description
| Format/Length: | max n3.max n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
ESTIMATED GLOMERULAR FILTRATION RATE is the result of the Clinical Investigation to determine the PATIENT's Estimated Glomerular Filtration Rate (eGFR), a test that is used to assess how well the kidneys are working.
ESTIMATED GLOMERULAR FILTRATION RATE is a measurement of how many millilitres (ml) of waste fluid the kidneys can filter from the blood in a minute, measured in 'Millilitres per Minute divided by 1.ESTIMATED GLOMERULAR FILTRATION RATE is a measurement of how many millilitres (ml) of waste fluid the kidneys can filter from the blood in a minute, where the UNIT OF MEASUREMENT is 'Millilitres per Minute divided by 1.73 Square Metres (ml/min/1.73m2)'.
For the Cancer Outcomes and Services Data Set: Urology, ESTIMATED GLOMERULAR FILTRATION RATE is collected once at PATIENT DIAGNOSIS.
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed Description
| Format/Length: | max n2.max n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed Description
| Format/Length: | max n3.max n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed Description
| Format/Length: | max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | n1.n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
For the Cancer Outcomes and Services Data Set, FORCED EXPIRATORY VOLUME IN 1 SECOND (ABSOLUTE AMOUNT) is presented in the range 0.10 to 9.99.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
For the Cancer Outcomes and Services Data Set, FORCED EXPIRATORY VOLUME IN 1 SECOND (PERCENTAGE) is presented in the range 1 to 150.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed Description
| Format/Length: | n2 |
| HES Item: | GESTAT |
| National Codes: | |
| Default Codes: | 99 - Not known |
Notes:
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
GESTATION LENGTH (AT BIRTH) is calculated as:
280 - (ESTIMATED DATE OF DELIVERY (AGREED) - PERSON BIRTH DATE (BABY)).
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed Description
| Format/Length: | n10 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:The total number of GENERAL PRACTITIONER written referrals, whether from doctors or dentists, received within the REPORTING PERIOD for a first Out-Patient Appointment Consultant regardless of whether or not they resulted in an Out-Patient Attendance Consultant.
It is the total number of GP written referrals where:GP WRITTEN REFERRALS is the total number of GP written referrals where:
| a. | the REFERRAL REQUEST TYPE of the REFERRAL REQUEST is National Code 'GP referral request' | ||
| and | |||
| b. | the WRITTEN REFERRAL REQUEST INDICATOR of the REFERRAL REQUEST is classification 'Yes' | ||
| and | |||
| c. | the REFERRAL REQUEST is for an Out-Patient Appointment Consultant whether directed to a specific CONSULTANT or not | ||
| and | |||
| d. | the ORIGINAL REFERRAL REQUEST RECEIVED DATE of the REFERRAL REQUEST is within the period of the REPORTING PERIOD START DATE and the REPORTING PERIOD END DATE. | ||
| Within the REPORTING PERIOD includes where the DATE is the same as the START DATE or END DATE | |||
Change to Data Element: Changed Description
| Format/Length: | n10 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
GP WRITTEN REFERRALS BOOKED is the total number of GP WRITTEN REFERRALS, whether from doctors or dentists, received within the REPORTING PERIOD for a first Out-Patient Appointment Consultant where a booking system was used. This is regardless of whether or not they resulted in an Out-Patient Attendance Consultant.
Currently this count only includes GP WRITTEN REFERRALS to a named CONSULTANT and excludes any other form of REFERRAL REQUEST whether to a named CONSULTANT or not.
It is the total number of GP WRITTEN REFERRALS where:GP WRITTEN REFERRALS BOOKED is the total number of GP WRITTEN REFERRALS where:
| a. | the REFERRAL REQUEST TYPE of the REFERRAL REQUEST is National Code 'GP referral request' | ||
| and | |||
| b. | the WRITTEN REFERRAL REQUEST INDICATOR of the REFERRAL REQUEST is classification 'Yes' | ||
| and | |||
| c. | the REFERRAL REQUEST is to a CONSULTANT for an Out-Patient Appointment Consultant | ||
| and | |||
| d. | the ORIGINAL REFERRAL REQUEST RECEIVED DATE of the REFERRAL REQUEST is within the period of the REPORTING PERIOD START DATE and the REPORTING PERIOD END DATE | ||
| Within the REPORTING PERIOD includes where the DATE is the same as the START DATE or END DATE | |||
| and | |||
| e. | the APPOINTMENT BOOKING SYSTEM TYPE of the REFERRAL REQUEST is classification 'Partial booking system non- Choose and Book system' or 'Full booking system - non Choose and Book system' or 'Choose and Book system' | ||
Out-Patient Appointment Consultant is a SERVICE REQUEST for an APPOINTMENT where APPOINTMENT CLASSIFICATION CODE is National Code 'Out-Patient Appointment Consultant'.
Change to Data Element: Changed Description
| Format/Length: | n10 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:The total number of APPOINTMENTS resulting from GENERAL PRACTITIONER written referrals, whether from doctors or dentists, where PATIENTS have been added to an Out-Patient Waiting List within the REPORTING PERIOD for a first Out-Patient Appointment Consultant.
Currently this count only includes GP written referrals to a named CONSULTANT and excludes any other form of REFERRAL REQUEST whether to a named CONSULTANT or not.
The DECISION TO OFFER AN APPOINTMENT DATE of a SERVICE REQUEST indicates in which REPORTING PERIOD the PATIENT was added to an Out-Patient Waiting List as a result of the REFERRAL REQUEST received. Note there may be a period of time between when the referral was recieved and when the decision to offer an appointment was made and recorded depending upon referral process and whether and what type of booking systems was used. Note there may be a period of time between when the referral was received and when the decision to offer an APPOINTMENT was made and recorded depending upon referral process and whether and what type of booking systems was used.
It is the total number of APPOINTMENTS arising from GP written referrals where:GP WRITTEN REFERRALS MADE is the total number of APPOINTMENTS arising from GP written referrals where:
| a. | the REFERRAL REQUEST TYPE of the REFERRAL REQUEST is National Code 'GP referral request' | ||
| and | |||
| b. | the WRITTEN REFERRAL REQUEST INDICATOR of the REFERRAL REQUEST is classification 'Yes' | ||
| and | |||
| c. | the REFERRAL REQUEST is to a CONSULTANT for an Out-Patient Appointment Consultant | ||
| and | |||
| d. | the DECISION TO OFFER AN APPOINTMENT DATE of the SERVICE REQUEST is within the period of the REPORTING PERIOD START DATE and the REPORTING PERIOD END DATE. | ||
| Within the REPORTING PERIOD includes where the DATE is the same as the START DATE or END DATE | |||
| and | |||
| e. | the APPOINTMENT FIRST ATTENDANCE of the APPOINTMENT is National Code 'First appointment' | ||
| or | |||
| where there is one or more APPOINTMENT recorded for a PATIENT but none has as yet taken place, the notional 'first appointment' included in the count will be the APPOINTMENT with the earliest APPOINTMENT DATE. This excludes any APPOINTMENTS which have been cancelled as indicated by a recorded APPOINTMENT CANCELLED DATE. | |||
Out-Patient Appointment Consultant is a SERVICE REQUEST for an APPOINTMENT where APPOINTMENT CLASSIFICATION CODE is National Code 02 'Out-Patient Appointment Consultant'.
Change to Data Element: Changed Description
| Format/Length: | n10 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:The total number of FIRST ATTENDANCE APPOINTMENTS resulting from GP WRITTEN REFERRALS, whether from doctors or dentists, where the Out-Patient Attendance Consultant took place within the REPORTING PERIOD.GP WRITTEN REFERRALS SEEN is the total number of FIRST ATTENDANCE APPOINTMENTS resulting from GP WRITTEN REFERRALS, whether from doctors or dentists, where the Out-Patient Attendance Consultant took place within the REPORTING PERIOD. This includes private PATIENTS attendances.
Currently this count only includes GP WRITTEN REFERRALS to a named CONSULTANT and excludes any other form of REFERRAL REQUEST whether to a named CONSULTANT or not.
When an Out-Patient Appointment Consultant APPOINTMENT takes place an Out-Patient Attendance Consultant CARE CONTACT records the attendance with FIRST ATTENDANCE recording whether it is a FIRST ATTENDANCE or a follow-up attendance and ACTIVITY DATE recording the Attendance Date.
The ADMINISTRATIVE CATEGORY records whether a PATIENT is a private or NHS PATIENT and should be the ADMINISTRATIVE CATEGORY which is current at the DATE of the attendance ACTIVITY DATE.
It is the total number of GP WRITTEN REFERRALS FIRST ATTENDANCE APPOINTMENTS where:GP WRITTEN REFERRALS SEEN is the total number of GP WRITTEN REFERRALS FIRST ATTENDANCE APPOINTMENTS where:
| a. | the REFERRAL REQUEST TYPE of the REFERRAL REQUEST is National Code 'GP referral request' | ||
| and | |||
| b. | the WRITTEN REFERRAL REQUEST INDICATOR of the REFERRAL REQUEST is classification 'Yes' | ||
| and | |||
| c. | the REFERRAL REQUEST is to a CONSULTANT for an Out-Patient Appointment Consultant | ||
| and | |||
| d. | the FIRST ATTENDANCE of the Out-Patient Attendance Consultant CARE CONTACT is National Code 'First attendance face to face' or 'First telephone or telemedicine consultation' | ||
| and | |||
| e. | the ACTIVITY DATE of the Out-Patient Attendance Consultant CARE CONTACT is within the period of the REPORTING PERIOD START DATE and the REPORTING PERIOD END DATE | ||
| Within the REPORTING PERIOD includes where the DATE is the same as the START DATE or END DATE | |||
Out-Patient Appointment Consultant is an Appointment Request for an APPOINTMENT where APPOINTMENT CLASSIFICATION CODE is National Code 02 'Out-Patient Appointment Consultant'.
Out-Patient Attendance Consultant is a CARE CONTACT where CARE CONTACT TYPE is National Code 30 'Out-Patient Attendance Consultant'.
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
For the Cancer Outcomes and Services Data Set, the value is presented in the range 1.0-25.0
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.max n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | n1.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.max n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed Description
| Format/Length: | max an2 |
| HES Item: | |
| National Codes: | |
| Default Codes: | NK - Invasive size not known NA - Size not applicable (non-invasive or micro-invasive cancer only) |
Notes:
INVASIVE TUMOUR SIZE is the same as attribute TUMOUR SIZE.
INVASIVE TUMOUR SIZE is the size of the Tumour in millimetres and is only applicable where the cancer detected was invasive.INVASIVE TUMOUR SIZE is the size of the Tumour, where the UNIT OF MEASUREMENT is 'Millimetres (mm)' and is only applicable where the cancer detected was invasive.
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
LESION SIZE (PATHOLOGICAL) is the same as attribute LESION SIZE.
LESION SIZE (PATHOLOGICAL) is the diameter of the Lesion, (or largest Lesion if there is more than one), where the histology of a SAMPLE proves to be invasive, measured in 'Millimetres (mm)'.LESION SIZE (PATHOLOGICAL) is the diameter of the Lesion, (or largest Lesion if there is more than one), where the histology of a SAMPLE proves to be invasive, where the UNIT OF MEASUREMENT is 'Millimetres (mm)'.
Change to Data Element: Changed Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
LESION SIZE (RADIOLOGICAL) is the same as attribute LESION SIZE.
LESION SIZE (RADIOLOGICAL) is the radiologically estimated size of the maximum diameter of the primary Lesion (or largest Lesion if there is more than one), measured in Millimetres (mm)'.LESION SIZE (RADIOLOGICAL) is the radiologically estimated size of the maximum diameter of the primary Lesion (or largest Lesion if there is more than one), where the UNIT OF MEASUREMENT is 'Millimetres (mm)'.
For the Cancer Outcomes and Services Data Set: Central Nervous System:
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3.max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3.max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3.max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
- 'Millilitres per Minute (ml/min)' where the measurement is uncorrected or
- 'Millilitres per Minute (ml/min/1.73m2)' where the measurement is corrected.
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.max n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
The value is presented in the range 0-20.
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.max n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
NON INVASIVE TUMOUR SIZE is the size of the non invasive Tumour and is only required if there is no invasive component.
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n4 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:NORMALISED PROTEIN CATABOLIC RATE (DIALYSIS) is the result of the Clinical Investigation which measures the PATIENT's normalised protein catabolic rate in "g/kg/day" to calculate the peritoneal dialysis clearance.
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed Description
| Format/Length: | n10 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
NUMBER OF OUT-PATIENT CONVERTED UNIQUE BOOKING REFERENCE NUMBERS is the total number of APPOINTMENTS where the APPOINTMENT ACCEPTED DATE is within the REPORTING PERIOD for an Out-Patient Appointment Consultant and where the Choose and Book system was used.
Currently this count only includes requests to a named CONSULTANT for an Out-Patient Appointment Consultant and excludes any other form of request.
The APPOINTMENT ACCEPTED DATE of an APPOINTMENT indicates in which REPORTING PERIOD the APPOINTMENT DATE OFFERED of an APPOINTMENT OFFER was accepted by, or on behalf of the PATIENT this is regardless of the APPOINTMENT DATE which may be within a different period.
When an APPOINTMENT is created and recorded, the unique booking reference used by the Choose and Book system is recorded by UNIQUE BOOKING REFERENCE NUMBER (CONVERTED).
It is the total of number of APPOINTMENTS where:
| a. | the REFERRAL REQUEST TYPE of the REFERRAL REQUEST is National Code 'GP referral request' | ||
| and | |||
| b. | the REFERRAL REQUEST is to a CONSULTANT for an Out-Patient Appointment Consultant | ||
| and | |||
| c. | the APPOINTMENT ACCEPTED DATE is within the period of the REPORTING PERIOD START DATE and the REPORTING PERIOD END DATE. | ||
| Within the REPORTING PERIOD includes where the DATE is the same as the Start Date or End Date | |||
| and | |||
| d. | the APPOINTMENT BOOKING SYSTEM TYPE of the APPOINTMENT is classification 'Choose and Book system' | ||
Out-Patient Appointment Consultant is a SERVICE REQUEST for an APPOINTMENT where APPOINTMENT CLASSIFICATION CODE is National Code 'Out-Patient Appointment Consultant'.
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (ALANINE AMINOTRANSFERASE CONCENTRATION) is the date when the PATIENT's alanine aminotranferase concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (ALKALINE PHOSPHATASE CONCENTRATION) is the date when the PATIENT's alkaline phosphatase concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:OBSERVATION DATE (ANTENATAL) is the same as attribute PERSON PROPERTY OBSERVED DATE, for a MEASURED PERSON OBSERVATION for the mother during the Antenatal period of a Maternity Episode.OBSERVATION DATE (ANTENATAL) is the same as attribute PERSON PROPERTY OBSERVED DATE for the mother during the Antenatal period of a Maternity Episode.
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (ASPARTATE AMINOTRANSFERASE CONCENTRATION) is the date when the PATIENT's aspartate aminotranferase concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (BILIRUBIN CONCENTRATION) is the date when the PATIENT's bilirubin concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (BLOOD GASES TEST) is the date when the PATIENT's blood gases test was taken.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (BLOOD PRESSURE) is the date when the PATIENT's Blood Pressure was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (BLOOD PRESSURE PRE-HAEMODIALYSIS) is the date when the PATIENT's pre-dialysis Blood Pressure was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (BLOOD TEST) is the date when the PATIENT's blood test was taken.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (BLOOD UREA CONCENTRATION) is the date when the PATIENT's blood urea concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | see DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:The PERSON PROPERTY OBSERVED DATE when the Body Mass Index was calculated.OBSERVATION DATE (BMI) is the same as attribute ACTIVITY DATE where the ACTIVITY DATE TYPE is National Code 'Clinical Intervention Date'.
OBSERVATION DATE (BMI) is the date when the Body Mass Index was calculated.
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (BONE AGE) is the date when the PATIENT's Bone Age was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (CALCULATED CREATININE CLEARANCE) is the date when the PATIENT's calculated creatinine clearance was measured.
OBSERVATION DATE (CALCULATED CREATININE CLEARANCE) is required for PATIENTS under 18 years only.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (CHEST X-RAY) is the date when the PATIENT's chest X-ray was taken.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (COMBINED Kt/V) is the date when the PATIENT's combined Kt/V was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (CORE ANTIBODY) is the date when the PATIENT's core antibody status was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (CYCLOSPORINE A 12 HOUR TROUGH LEVEL) is the date when the recipient's cyclosporine A 12 hour trough level was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (CYCLOSPORINE A 2 HOUR LEVEL C2) is the date when the recipient's cyclosporine A 2 hour level (C2) was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (CYTOMEGALOVIRUS) is the date when the PATIENT's Cytomegalovirus status was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (CYTOMEGALOVIRUS POLYMERASE CHAIN REACTION VIRAL LOAD) is the date when the PATIENT's Cytomegalovirus Polymerase Chain Reaction viral load was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (DIALYSATE 24 HOUR CREATININE CONCENTRATION) is the date when the PATIENT's 24 hour dialysate creatinine concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (DIALYSATE 24 HOUR PROTEIN LOSS) is the date when the PATIENT's 24 hour dialysate protein loss was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (DIALYSATE 24 HOUR UREA CONCENTRATION) is the date when the PATIENT's 24 hour dialysate urea concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (DIALYSATE 24 HOUR VOLUME) is the date when the PATIENT's measured 24 hour dialysate volume was taken.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (DIALYSATE Kt/V) is the date when the PATIENT's dialysate Kt/V was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (ELECTROCARDIOGRAM) is the date when the PATIENT's Electrocardiogram was taken.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (EPSTEIN-BARR VIRUS) is the date when the PATIENT's Epstein-Barr virus status was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (ESTIMATED GLOMERULAR FILTRATION RATE) is the date when the PATIENT's estimated glomerular filtration rate was taken.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | see DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:The date when the DIABETES ROUTINE REVIEW (EYE) took place.
OBSERVATION DATE (EYE EXAMINATION) is the same as attribute PERSON PROPERTY OBSERVED DATE.OBSERVATION DATE (EYE EXAMINATION) is the date when the DIABETES ROUTINE REVIEW (EYE) took place.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | see DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:The date when the DIABETES ROUTINE REVIEW (FOOT) took place.
OBSERVATION DATE (FOOT EXAMINATION) is the same as attribute PERSON PROPERTY OBSERVED DATE.OBSERVATION DATE (FOOT EXAMINATION) is the date when the DIABETES ROUTINE REVIEW (FOOT) took place.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (FULL BLOOD COUNT TEST) is the date when the PATIENT's full blood count test was taken.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (GAMMA GLUTAMYL TRANSFERASE CONCENTRATION) is the date when the PATIENT's gamma glutamyl transferase concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (GRAFT CLINICAL ASSESSMENT) is the date of the clinical assessment of the functioning graft.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (HAEMOGLOBIN CONCENTRATION) is the date when the PATIENT's haemoglobin concentration level was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (HbA1c LEVEL) is the date when the HbA1c level was taken.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (HEAD CIRCUMFERENCE) is the date when the PATIENT's Head Circumference was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (HEIGHT) is the date when the PATIENT's Height was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (HEPATITIS B ANTIBODY) is the date when the PATIENT's Hepatitis B surface antibody status was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (HEPATITIS B ANTIGEN) is the date when the PATIENT's Hepatitis B surface antigen status was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (HEPATITIS B E ANTIBODY) is the date when the PATIENT's Hepatitis B E surface antibody status was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (HEPATITIS C ANTIBODY) is the date when the PATIENT's Hepatitis C surface antibody status was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (HIGH DENSITY LIPOPROTEIN CHOLESTEROL CONCENTRATION) is the date when the PATIENT's high density lipoprotein cholesterol concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (HUMAN IMMUNODEFICIENCY VIRUS) is the date when the PATIENT's human immunodeficiency virus status was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (HYPOCHROMIC RED CELLS PERCENTAGE) is the date when the PATIENT's hypochromic red cells percentage was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (LACTATE DEHYDROGENASE CONCENTRATION) is the date when the PATIENT's lactate dehydrogenase concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (LOW DENSITY LIPOPROTEIN CHOLESTEROL CONCENTRATION) is the date when the PATIENT's low density lipoprotein cholesterol concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (MEASURED 24 HOUR CREATININE CLEARANCE) is the date when the PATIENT's measured 24 hour creatinine clearance was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (MEASURED CREATININE CLEARANCE) is the date when the PATIENT's measured creatinine clearance was taken.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (MEASURED GLOMERULAR FILTRATION RATE) is the date when the PATIENT's measured glomerular filtration rate was taken.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (MYCOPHENOLIC ACID TROUGH LEVEL) is the date when the recipient's mycophenolic acid trough level was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (NET DAILY ULTRAFILTRATION) is the date when the PATIENT's net daily ultrafiltration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (NORMALISED PROTEIN CATABOLIC RATE) is the date when the PATIENT's normalised protein catabolic rate was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (NORMALISED WEEKLY PERITONEAL CREATININE CLEARANCE) is the date when the PATIENT's weekly peritoneal dialysis normalised creatinine clearance was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (PERITONEAL DIALYSIS TOTAL WEEKLY FLUID VOLUME) is the date when the PATIENT's total weekly fluid volume on peritoneal dialysis was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (PERITONEAL EQUILIBRATION TEST) is the date of the Peritoneal Equilibration Test.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (PHOSPHATE CONCENTRATION) is the date when the PATIENT's phosphate concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (PLATELETS COUNT) is the date when the PATIENT's platelets count was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (PROTEIN CREATININE RATIO) is the date when the recipient's protein:creatinine ratio was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (RED CELL FOLATE CONCENTRATION) is the date when the PATIENT's red cell folate concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (RESIDUAL RENAL CREATININE CLEARANCE) is the date when the PATIENT's weekly urinary creatinine clearance was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (RESIDUAL URINE OUTPUT) is the date when the PATIENT's residual urine output was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (SERUM ALBUMIN CONCENTRATION) is the date when the PATIENT's serum albumin concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (SERUM ALUMINIUM CONCENTRATION) is the date when the PATIENT's serum aluminium concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (SERUM B12 CONCENTRATION) is the date when the PATIENT's serum B12 concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (SERUM BICARBONATE CONCENTRATION) is the date when the PATIENT's serum bicarbonate concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (SERUM CALCIUM CONCENTRATION) is the date when the PATIENT's serum calcium concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | see DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:The PERSON PROPERTY OBSERVED DATE for the MEASURED PERSON OBSERVATION of the type 'Serum Cholesterol Level'.OBSERVATION DATE (SERUM CHOLESTEROL LEVEL) is the is the same as attribute ACTIVITY DATE where the ACTIVITY DATE TYPE is National Code 'Clinical Intervention Date'.
OBSERVATION DATE (SERUM CHOLESTEROL LEVEL) is the date when the Serum Cholesterol Level was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (SERUM C-REACTIVE PROTEIN CONCENTRATION) is the date when the PATIENT's serum C-reactive protein concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (SERUM CREATININE CONCENTRATION) is the date when the PATIENT's serum creatinine concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (SERUM CREATININE Kt/V) is the date when the PATIENT's serum creatinine Kt/V was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | see DATE |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:The PERSON PROPERTY OBSERVED DATE for the MEASURED PERSON OBSERVATION of the type 'Serum Creatinine Level'.OBSERVATION DATE (SERUM CREATININE LEVEL) is the same as attribute ACTIVITY DATE where the ACTIVITY DATE TYPE is National Code 'Clinical Intervention Date'.
OBSERVATION DATE (SERUM CREATININE LEVEL) is the date when the Serum Creatinine Level was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (SERUM FERRITIN CONCENTRATION) is the date when the PATIENT's serum ferritin concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (SERUM INTACT PARATHYROID HORMONE CONCENTRATION) is the date when the PATIENT's serum intact parathyroid hormone concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (SERUM MAGNESIUM CONCENTRATION) is the date when the PATIENT's serum magnesium concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (SERUM POTASSIUM CONCENTRATION) is the date when the PATIENT's serum potassium concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (SIROLIMUS TROUGH LEVEL) is the date when the recipient's sirolimus trough level was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (TACROLIMUS 12 HOUR TROUGH LEVEL) is the date when the recipient's tacrolimus 12 hour trough level was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (TISSUE TYPING DONOR) is the date when the deceased ORGAN OR TISSUE DONOR's tissue typing (Human Leukocyte Antigen (HLA) report) was taken.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (TISSUE TYPING RECIPIENT) is the date when the recipient's tissue typing (Human Leukocyte Antigen (HLA) report) was taken.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (TOTAL CHOLESTEROL CONCENTRATION) is the date when the PATIENT's total cholesterol concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (TRANSFERRIN SATURATION) is the date when the PATIENT's transferrin saturation was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (TRIGLYCERIDES CONCENTRATION) is the date when the PATIENT's triglycerides concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (URIC ACID CONCENTRATION) is the date when the PATIENT's uric acid concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | see DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:The PERSON PROPERTY OBSERVED DATE for the MEASURED PERSON OBSERVATION of the type 'Urinary Albumin Level' .OBSERVATION DATE (URINARY ALBUMIN LEVEL) is the same as attribute ACTIVITY DATE where the ACTIVITY DATE TYPE is National Code 'Clinical Intervention Date'.
OBSERVATION DATE (URINARY ALBUMIN LEVEL) is the date when the Urinary Albumin Level was taken.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (URINE CREATININE CONCENTRATION) is the date when the PATIENT's urine creatinine concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (URINE DIPSTICK TEST BLOOD) is the date when the PERSON's urine dipstick test for blood was taken.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (URINE DIPSTICK TEST PROTEIN) is the date when the PATIENT's urine dipstick test for protein was taken.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (URINE Kt/V) is the date when the PATIENT's urine kt/v was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (URINE UREA CONCENTRATION) is the date when the PATIENT's urine urea concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (URINE VOLUME) is the date when the PATIENT's urine volume was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (VARICELLA-ZOSTER) is the date when the PERSON's Varicella-Zoster virus status was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (VITAMIN D CONCENTRATION) is the date when the PATIENT's vitamin D concentration was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (WAIST MEASUREMENT) is the date when the PATIENT's Waist Measurement was taken.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (WEIGHT) is the date when the PATIENT's Weight was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (WHITE BLOOD CELL COUNT) is the date when the PATIENT's white blood cell count was taken.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (WHOLE BLOOD MEAN CELL VOLUME) is the date when the PATIENT's whole blood mean cell volume (MCV) was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
OBSERVATION DATE (WHOLE BLOOD MEAN CORPUSCULAR HAEMOGLOBIN) is the date when the PATIENT's whole blood mean corpuscular haemoglobin (MCH) was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE AND TIME |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE AND TIME |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:OBSERVATION DATE AND TIME (BLOOD PRESSURE AVERAGED) is the same as data element PERSON OBSERVATION DATE AND TIME of the PERSON's average Blood Pressure.OBSERVATION DATE AND TIME (BLOOD PRESSURE AVERAGED) is the same as attribute ACTIVITY DATE and ACTIVITY TIME where the ACTIVITY DATE AND TIME TYPE is National Code 'Clinical Intervention Date and Time'.
OBSERVATION DATE AND TIME (BLOOD PRESSURE AVERAGED) is the date and time when the PERSON's average Blood Pressure was measured.
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE AND TIME |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE AND TIME |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE AND TIME |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE AND TIME |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE AND TIME |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE AND TIME |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE AND TIME |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | See DATE AND TIME |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed Description
| Format/Length: | an3 or an5 |
| HES Item: | PURCODE |
| National Codes: | |
| ODS Default Codes: | VPP00 - Private PATIENTS / Overseas Visitor liable for charge |
| XMD00 - Commissioner Code for Ministry of Defence (MoD) Healthcare | |
| YDD82 - Episodes funded directly by the National Commissioning Group for England |
Notes:
ORGANISATION CODE (CODE OF COMMISSIONER) is the same as attribute ORGANISATION CODE.
ORGANISATION CODE (CODE OF COMMISSIONER) is the ORGANISATION CODE of the ORGANISATION commissioning health care.
For Commissioning Data Sets, the ORGANISATION CODE (CODE OF COMMISSIONER) should always be the ORGANISATION CODE of the original commissioner to support Payment by Results.
The NHS England document "Who pays?The NHS England document "Who pays? Determining responsibility for payments to providers" sets out a framework for establishing responsibility for commissioning an individual's care within the NHS, (i.e. determining who pays for a PATIENT’s care.)
The document includes information on the following:
- General Rules
- Applying the rules to Clinical Commissioning Group commissioned services
- Exceptions to the general rules
- Examples to help clarify the boundaries of responsibility between commissioning ORGANISATIONS.
For further information on this document contact NHS England at "Contact us".
Change to Data Element: Changed Description
| Format/Length: | an3 |
| HES Item: | PCTR |
| National Codes: | |
| ODS Default Codes: | Q99 - High Level Health Geography/Primary Care ORGANISATION of Residence Not Known Note: this code must not be used in the Commissioning Data Set (CDS) header. It is not a default Commissioner code. |
| X98 - Primary Care ORGANISATION Not Applicable (Overseas Visitors) Note: this code must not be used in the Commissioning Data Set (CDS) header. It is not a default Commissioner code. |
Notes:
ORGANISATION CODE (PCT OF RESIDENCE) is the same as attribute ORGANISATION CODE.
ORGANISATION CODE (PCT OF RESIDENCE) is the ORGANISATION CODE derived from the PATIENT's POSTCODE OF USUAL ADDRESS, where they reside within the boundary of a:
- Northern Ireland Local Commissioning Group Guidance on the use of Northern Ireland codes can be found in Data Set Change Notice 19/2009
ORGANISATION CODES can be downloaded from the Organisation Data Service website or through the online Technology Reference Data Update Distribution Service (TRUD).ORGANISATION CODES can be downloaded from the Organisation Data Service website or through the online Technology Reference Data Update Distribution Service (TRUD). For further information, see Organisation Data Service.
For PATIENTS who are Overseas Visitors: Organisation Data Service Default Code X98 'Primary Care Organisation Not Applicable (Overseas Visitors) should be reported.
Note: A review of Organisation Data Service Default Codes is planned to be carried out and this default code will be updated as part of that.
For the purposes of sending Commissioning Data Set messages to the Secondary Uses Service (regardless of how local systems hold the data), it is essential at present to continue using a 3 character field, using the first 3 characters of the ORGANISATION CODE (PCT OF RESIDENCE) and following the same update rules relating to Prime Recipient as are currently in place. This is necessary, primarily to preserve the integrity of the current Commissioning Data Set message and the CDS PRIME RECIPIENT IDENTITY which is derived from the ORGANISATION CODE (PCT OF RESIDENCE).
At April 2013, Primary Care Trusts will no longer exist. Health Care Providers still using Commissioning Data Set version 6-1 (CDS-XML schema 6-1-1) at this point must complete the ORGANISATION CODE (PCT OF RESIDENCE) field in the PATIENT IDENTITY group structures, with the data which is carried in ORGANISATION CODE (RESIDENCE RESPONSIBILITY) in Commissioning Data Set version 6-2 (generally, this will be the Clinical Commissioning Group (CCG) of Residence, where the CCG has taken over the responsibilities of the Primary Care Trust).
The Organisation Data Service provides postcode files which link postcodes to the Primary Care Trust. See NHS Postcode Directory.
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3.max n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.max n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
In a Nutritional Assessment for a renal PATIENT this would be measured over a 3 month period.For the National Renal Data Set, during a Nutritional Assessment for a renal PATIENT, PERCENTAGE WEIGHT LOSS is measured over a 3 month period.
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3.max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | n1.max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
For the Systemic Anti-Cancer Therapy Data Set, PERSON HEIGHT IN METRES is the Height at the start of the Systemic Anti-Cancer Drug Regimen.
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | n2.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | n4 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
For the Diabetes Data Set (Summary Core), PERSON OBSERVATION (URINARY ALBUMIN LEVEL) must be accompanied by a recorded URINARY ALBUMIN LEVEL TESTING METHOD.
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3.max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Notes:
For the Commissioning Data Sets, PERSON WEIGHT must be padded to match the Format/Length pattern of n3.n3, for example 001.100 is a valid entry (1.1 is invalid)
For Neonatal Critical Care Minimum Data Set, PERSON WEIGHT will be the last recorded Weight on a particular ACTIVITY DATE (CRITICAL CARE)
- For the Systemic Anti-Cancer Therapy Data Set, PERSON WEIGHT is recorded at the start of the:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3.max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3.max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3.max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n4 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | n1.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | n1.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed Description
| Format/Length: | max n2.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed Description
| Format/Length: | max n5 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
PRESCRIBED ITEM (VOLUME OF 136 GLUCOSE FLUID) is the volume used per day of the RENAL DIALYSIS MEDICATION TYPE '1.
Change to Data Element: Changed Description
| Format/Length: | max n5 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
PRESCRIBED ITEM (VOLUME OF 227 GLUCOSE FLUID) is the volume used per day of the RENAL DIALYSIS MEDICATION TYPE '2.
Change to Data Element: Changed Description
| Format/Length: | max n5 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
PRESCRIBED ITEM (VOLUME OF 386 GLUCOSE FLUID) is the volume used per day of the RENAL DIALYSIS MEDICATION TYPE '3.
Change to Data Element: Changed Description
| Format/Length: | max n4 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed Description
| Format/Length: | max n4 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n1.max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed Description
| Format/Length: | max n5 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
For the National Renal Data Set, PRESCRIBED TOTAL DAILY DOSE (ALEMTUZUMAB) is the prescribed daily amount being administered to the PATIENT at the time of the follow up assessment.
Change to Data Element: Changed Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
For the National Renal Data Set, PRESCRIBED TOTAL DAILY DOSE (AZATHIOPRINE) is the prescribed daily amount being administered to the PATIENT at the time of the follow up assessment.
Change to Data Element: Changed Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
For the National Renal Data Set, PRESCRIBED TOTAL DAILY DOSE (CICLOSPORIN) is the prescribed daily amount being administered to the PATIENT at the time of the follow up assessment.
Change to Data Element: Changed Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
For the National Renal Data Set, PRESCRIBED TOTAL DAILY DOSE (DACLIZUMAB) is the prescribed daily amount being administered to the PATIENT at the time of the follow up assessment.
Change to Data Element: Changed Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
For the National Renal Data Set, PRESCRIBED TOTAL DAILY DOSE (MYCOPHENOLATE SODIUM) is the prescribed daily amount being administered to the PATIENT at the time of the follow up assessment.
Change to Data Element: Changed Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
For the National Renal Data Set, PRESCRIBED TOTAL DAILY DOSE (TACROLIMUS) is the prescribed daily amount being administered to the PATIENT at the time of the follow up assessment.
Change to Data Element: Changed Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
PRIMARY TUMOUR SIZE (RADIOLOGICAL) is the same as attribute TUMOUR SIZE.
PRIMARY TUMOUR SIZE (RADIOLOGICAL) is the maximum dimension of the primary Tumour, as agreed at the Multidisciplinary Team Meeting, in 'Millimetres (mm)'.PRIMARY TUMOUR SIZE (RADIOLOGICAL) is the maximum dimension of the primary Tumour, as agreed at the Multidisciplinary Team Meeting, where the UNIT OF MEASUREMENT is 'Millimetres (mm)'.
Change to Data Element: Changed Description
| Format/Length: | See DATE |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
PROCEDURE DATE (STEM CELL INFUSION) is the same as data element PROCEDURE DATE.
PROCEDURE DATE (STEM CELL INFUSION) is the DATE of the Stem Cell Infusion.
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n5.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n5.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3.max n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed Description
| Format/Length: | n4 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed Description
| Format/Length: | n4 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed Description
| Format/Length: | max n3.n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
RADIOTHERAPY TOTAL DOSE is the total actual absorbed radiation dose received during a course of treatment.
For the Cancer Outcomes and Services Data Set: Core, RADIOTHERAPY TOTAL DOSE is derived from the Radiotherapy Data Set.
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | n1.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3.max n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n4 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.max n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | n1.max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n4 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n4.max n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | n1.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed Description
| Format/Length: | max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
The value is presented in the range 0-50.
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Change to Data Element: Changed linked Attribute
Change to Data Element: Changed linked Attribute
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.max n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed Description
| Format/Length: | n2 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:The method used to determine the PERSON OBSERVATION (URINARY ALBUMIN LEVEL).
The urine specimen used to check for albuminuria may be collected in several ways depending on local preference. Staging definitions vary by method so PERSON OBSERVATION (URINARY ALBUMIN LEVEL) must be accompanied by the method used.
Derive from the MEASURED OBSERVATION VALUE recorded for the MEASURED PERSON OBSERVATION TYPE 'Urinary Albumin level'.
Permitted National Codes:
| 01 | Albumin concentration (mg/L) |
| 02 | Albumin creatinine ratio (mg/mmol) |
| 03 | Timed overnight albumin (µg/min) |
| 04 | 24hr albumin excretion (mg/24hr) |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n5 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n4 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n6 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
For the National Renal Data Set, URINE VOLUME is the PATIENT's urine volume by 24 hour collection.
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n7 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
For the HIV and AIDS Reporting Data Set, VIRAL LOAD COUNT should be omitted if the PATIENT's viral load count has not been recorded.
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n3.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n4 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed linked Attribute, Description
| Format/Length: | max n2.n1 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
Change to Data Element: Changed linked Attribute, Description
Attribute:
| CLINICAL INVESTIGATION RESULT VALUE |
Change to Data Element: Changed Description
| Format/Length: | max n3 |
| HES Item: | |
| National Codes: | |
| Default Codes: |
Notes:
WHOLE TUMOUR SIZE is the whole size of the Tumour and is only required where the Tumour has a DUCTAL CARCINOMA IN SITU GRADE.
For enquiries about this Change Request, please email information.standards@hscic.gov.uk