| Type: | Patch |
| Reference: | 1762 |
| Version No: | 1.0 |
| Subject: | April 2020 Release Patch |
| Effective Date: | Immediate |
| Reason for Change: | Patch |
| Publication Date: | 24 April 2020 |
Background:
This patch updates the NHS Data Model and Dictionary in preparation for the new release and includes:
- What's New amended to include Change Requests incorporated since the last version of the NHS Data Model and Dictionary was published
- Missing hyperlinks added
- Spelling mistakes corrected
- Website links updated
- HTML format corrected
- Updates to / retirement of items in preparation for the new version of the dictionary.
To view a demonstration on "How to Read an NHS Data Model and Dictionary Change Request", visit the NHS Data Model and Dictionary help pages at: https://www.datadictionary.nhs.uk/Flash_Files/changerequest.htm.
Note: if the web page does not open, please copy the link and paste into the web browser.
Summary of changes:
| Date: | 24 April 2020 |
| Sponsor: | Nicholas Oughtibridge, Head of Clinical Data Architecture, NHS Digital |
Note: New text is shown with a blue background. Deleted text is crossed out. Retired text is shown in grey. Within the Diagrams deleted classes and relationships are red, changed items are blue and new items are green.
Click here for a printer friendly view of this page.
Change to Data Set: Changed Description
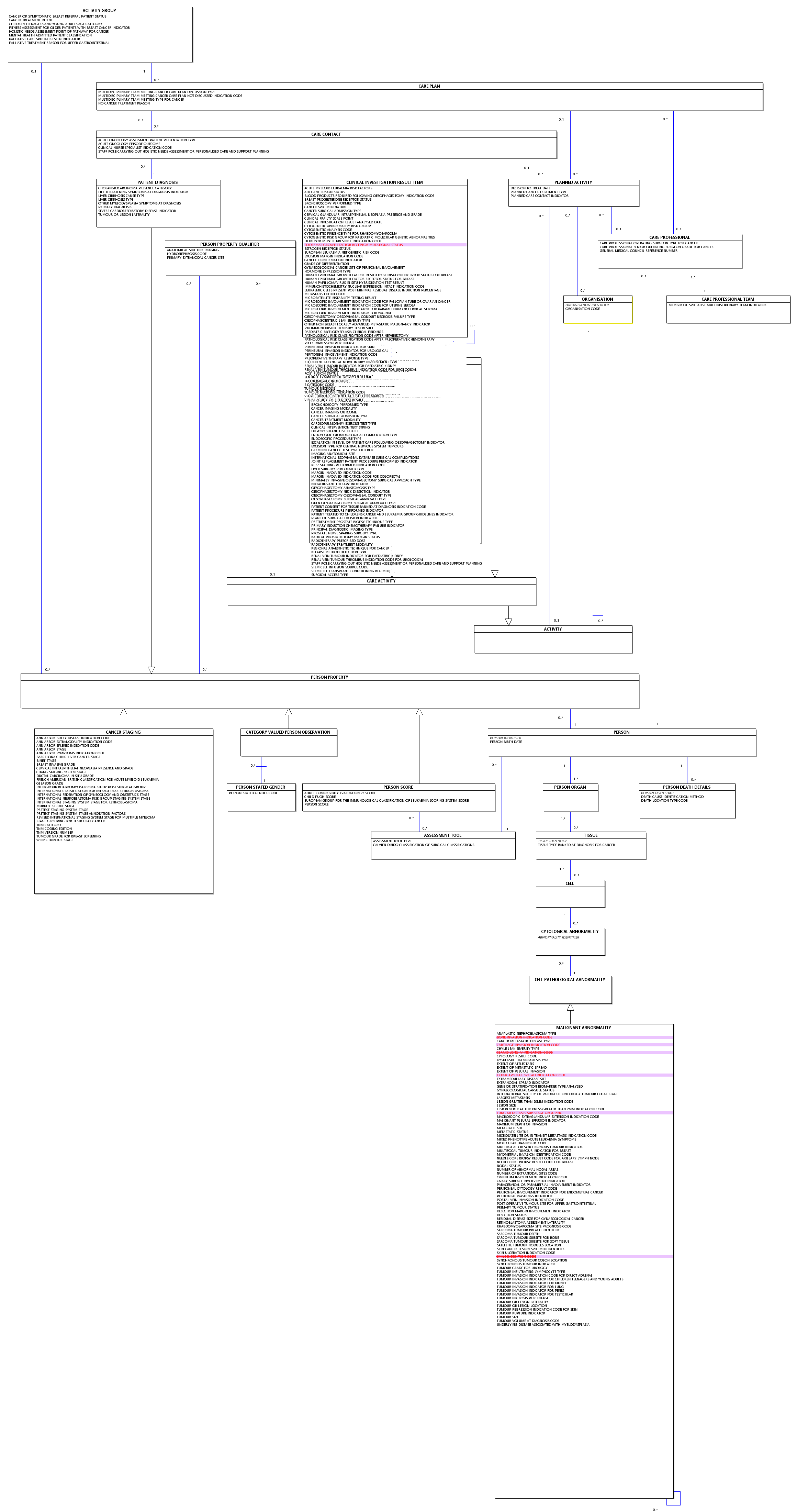
Cancer Outcomes and Services Data Set Overview
Due to the rapidly changing situation with Covid-19, the implementation date of the Cancer Outcomes and Services Data Set (COSDS) has been deferred until 1 July 2020.
This will be reviewed and continued to be moved forward 3 months until there is a stable position nationally to resume the upgrade process.
For further information please contact: COSD@phe.gov.uk.
The previous version of the data set can be found at: COSDS Data Sets.
For a "Full Screen" view, click Cancer Outcomes and Services Data Set: Breast.
In the "Full Screen" view, to return to the "Data Set" view, click the browser "back" button.
The Mandatory or Required (M/R) column in the COSDS XML Schema indicates the recommendation for the inclusion of data.
- M = Mandatory: this data element is mandatory and the technical process (e.g. submission of the data set, production of output etc.) cannot be completed without this data element being present
- R = Required: NHS business processes cannot be delivered without this data element.
For guidance on downloading the XML Schema, see XML Schema TRUD Download.
For guidance on the XML Schema constraints, see the Cancer Outcomes and Services Data Set XML Schema Constraints.
| TRIPLE DIAGNOSTIC ASSESSMENT - BREAST |
|---|
| To carry diagnostic details for Breast cancer. One occurrence of this group is permitted. | |
| M/R | Data Set Data Elements |
| M | BREAST TRIPLE DIAGNOSTIC ASSESSMENT INDICATOR |
| PROGNOSTIC INDEX - BREAST |
|---|
| To carry prognostic details for Breast cancer. One occurrence of this group is permitted. | |
| M/R | Data Set Data Elements |
| M | NOTTINGHAM PROGNOSTIC INDEX SCORE |
| CLINICAL NURSE SPECIALIST AND RISK FACTOR ASSESSMENT: NATIONAL AUDIT OF BREAST CANCER IN OLDER PATIENTS (NABCOP) - BREAST |
|---|
| To carry National Audit of Breast Cancer in Older Patients assessment details for Breast cancer. One occurrence of this group is permitted per Core Clinical Nurse Specialist and Risk Factor Assessment. | |
| M/R | Data Set Data Elements |
| R | FITNESS ASSESSMENT FOR OLDER PATIENTS WITH BREAST CANCER INDICATOR |
| R | FITNESS ASSESSMENT FOR OLDER PATIENTS WITH BREAST CANCER COMPLETED DATE |
| R | CLINICAL FRAILTY SCALE POINT |
| R | ABBREVIATED MENTAL TEST SCORE |
| R | SEVERE CARDIORESPIRATORY DISEASE INDICATOR |
| R | OTHER NON BREAST LOCALLY ADVANCED METASTATIC MALIGNANCY INDICATOR |
Change to Data Set: Changed Description
Cancer Outcomes and Services Data Set Overview
Due to the rapidly changing situation with Covid-19, the implementation date of the Cancer Outcomes and Services Data Set (COSDS) has been deferred until 1 July 2020.
This will be reviewed and continued to be moved forward 3 months until there is a stable position nationally to resume the upgrade process.
For further information please contact: COSD@phe.gov.uk.
The previous version of the data set can be found at: COSDS Data Sets.
For a "Full Screen" view, click Cancer Outcomes and Services Data Set: Central Nervous System.
In the "Full Screen" view, to return to the "Data Set" view, click the browser "back" button.
The Mandatory or Required (M/R) column in the COSDS XML Schema indicates the recommendation for the inclusion of data.
- M = Mandatory: this data element is mandatory and the technical process (e.g. submission of the data set, production of output etc.) cannot be completed without this data element being present
- R = Required: NHS business processes cannot be delivered without this data element.
For guidance on downloading the XML Schema, see XML Schema TRUD Download.
For guidance on the XML Schema constraints, see the Cancer Outcomes and Services Data Set XML Schema Constraints.
| IMAGING - CENTRAL NERVOUS SYSTEM (CNS) |
|---|
| To carry imaging details for Central Nervous System (CNS) cancer. One occurrence of this group is permitted per Core Imaging. | |
| M/R | Data Set Data Elements |
| R | LESION LOCATION (RADIOLOGICAL) |
| R | NUMBER OF LESIONS (RADIOLOGICAL) |
| R | LESION SIZE (RADIOLOGICAL) |
| R | PRINCIPAL DIAGNOSTIC IMAGING TYPE |
| CANCER CARE PLAN - CENTRAL NERVOUS SYSTEM (CNS) |
|---|
| To carry cancer care plan details for Central Nervous System (CNS) cancer. One occurrence of this group is permitted Core Cancer Care Plan. | |
| M/R | Data Set Data Elements |
| R | PROVISIONAL DIAGNOSIS (ICD) |
| SURGERY - TREATMENT - CENTRAL NERVOUS SYSTEM (CNS) |
|---|
| To carry surgery details for Central Nervous System (CNS) cancer. One occurrence of this group is permitted per Core Treatment Surgery. | |
| M/R | Data Set Data Elements |
| R | TUMOUR LOCATION (SURGICAL) |
| R | BIOPSY TYPE (CENTRAL NERVOUS SYSTEM TUMOURS) |
| R | EXCISION TYPE (CENTRAL NERVOUS SYSTEM TUMOURS) |
| SURGERY - TREATMENT: CHILDREN, TEENAGERS AND YOUNG ADULTS (CTYA) - CENTRAL NERVOUS SYSTEM (CNS) |
|---|
| To carry surgery details for Children, Teenagers and Young Adults (CTYA) for Central Nervous System (CNS) cancer. One occurrence of this group is permitted per Core Treatment Surgery. | |
| M/R | Data Set Data Elements |
| R | RESECTION STATUS |
| DIAGNOSIS: LOW GRADE GLIOMA - CENTRAL NERVOUS SYSTEM (CNS) |
|---|
| To carry diagnostic Low Grade Glioma details for Central Nervous System (CNS) cancer. One occurrence of this group is permitted per Core Diagnosis. | |
| M/R | Data Set Data Elements |
| R | VISUAL ACUITY TEST RESULT (AT DIAGNOSIS) Multiple occurrences of this item are permitted |
| R | VISUAL FIELD TEST RESULT (AT DIAGNOSIS) Multiple occurrences of this item are permitted |
| SITE SPECIFIC STAGING: CEREBROSPINAL FLUID (CSF) - CENTRAL NERVOUS SYSTEM (CNS) |
|---|
| To carry site specific staging Cerebrospinal Fluid (CSF) details for Central Nervous System (CNS) cancer. One occurrence of this group is required per Core Site Specific Staging. | |
| M/R | Data Set Data Elements |
| M | CHANG STAGING SYSTEM STAGE |
| LABORATORY RESULTS: GERM CELL CENTRAL NERVOUS SYSTEM (CNS) TUMOURS CHOICE - CENTRAL NERVOUS SYSTEM (CNS) |
|---|
| One occurrence of this group is permitted per Core Laboratory Results One of the following Germ Cell Central Nervous System (CNS) tumour laboratory result details MUST be provided per Core Laboratory Results | |
| CHOICE 1 - ALPHA FETOPROTEIN | |
| To carry Germ Cell Central Nervous System (CNS) tumour laboratory result details. One occurrence of this group is required per Core Laboratory Results if selected as the choice. | |
| M/R | Data Set Data Elements |
| M | ALPHA FETOPROTEIN (CEREBROSPINAL FLUID) |
| CHOICE 2 - BETA HUMAN CHORIONIC GONADOTROPIN | |
| To carry Germ Cell Central Nervous System (CNS) tumour laboratory result details. One occurrence of this group is required per Core Laboratory Results if selected as the choice. | |
| M/R | Data Set Data Elements |
| M | BETA HUMAN CHORIONIC GONADOTROPIN (CEREBROSPINAL FLUID) |
Change to Data Set: Changed Description
Cancer Outcomes and Services Data Set Overview
Due to the rapidly changing situation with Covid-19, the implementation date of the Cancer Outcomes and Services Data Set (COSDS) has been deferred until 1 July 2020.
This will be reviewed and continued to be moved forward 3 months until there is a stable position nationally to resume the upgrade process.
For further information please contact: COSD@phe.gov.uk.
The previous version of the data set can be found at: COSDS Data Sets.
For a "Full Screen" view, click Cancer Outcomes and Services Data Set: Children, Teenagers and Young Adults.
In the "Full Screen" view, to return to the "Data Set" view, click the browser "back" button.
The Mandatory or Required (M/R) column in the COSDS XML Schema indicates the recommendation for the inclusion of data.
- M = Mandatory: this data element is mandatory and the technical process (e.g. submission of the data set, production of output etc.) cannot be completed without this data element being present
- R = Required: NHS business processes cannot be delivered without this data element.
For guidance on downloading the XML Schema, see XML Schema TRUD Download.
For guidance on the XML Schema constraints, see the Cancer Outcomes and Services Data Set XML Schema Constraints.
| REFERRALS - CHILDREN, TEENAGERS AND YOUNG ADULTS (CTYA) |
|---|
| To carry referral details for Children, Teenagers and Young Adults (CTYA) cancer. One occurrence of this group is permitted per Core Referrals and First Stage of Patient Pathway. | |
| M/R | Data Set Data Elements |
| R | CARE PROFESSIONAL MAIN SPECIALTY CODE (CANCER REFERRAL) |
| DIAGNOSIS - CHILDREN, TEENAGERS AND YOUNG ADULTS (CTYA) |
|---|
| To carry diagnostic details for Children, Teenagers and Young Adults (CTYA) cancer. One occurrence of this group is permitted per Core Diagnosis. | |
| M/R | Data Set Data Elements |
| R | CARE PROFESSIONAL MAIN SPECIALTY CODE (DIAGNOSIS) |
| R | CHILDREN TEENAGERS AND YOUNG ADULTS AGE CATEGORY (CONSULTANT AT DIAGNOSIS) |
| DIAGNOSIS: NEUROBLASTOMA - CHILDREN, TEENAGERS AND YOUNG ADULTS (CTYA) |
|---|
| To carry diagnostic Neuroblastoma details for Children, Teenagers and Young Adults (CTYA) cancer. One occurrence of this group is permitted per Core Diagnosis. | |
| M/R | Data Set Data Elements |
| R | LIFE THREATENING SYMPTOMS AT DIAGNOSIS INDICATOR (NEUROBLASTOMA) |
| SITE SPECIFIC STAGING CHOICE - CHILDREN, TEENAGERS AND YOUNG ADULTS (CTYA) |
|---|
| One of the following Site Specific Staging Sections MUST be provided per Core Site Specific Staging | |
| CHOICE 1 - WILMS | |
| To carry staging renal tumour details for Children, Teenagers and Young Adults (CTYA) cancer. One occurrence of this group is required per Core Site Specific Staging group if selected as the choice. | |
| M/R | Data Set Data Elements |
| M | WILMS TUMOUR STAGE |
| CHOICE 2 - INTERNATIONAL NEUROBLASTOMA RISK GROUP | |
| To carry staging Neuroblastoma details for Children, Teenagers and Young Adults (CTYA) cancer. One occurrence of this group is required per Core Site Specific Staging group if selected as the choice. | |
| M/R | Data Set Data Elements |
| M | INTERNATIONAL NEUROBLASTOMA RISK GROUP STAGING SYSTEM STAGE |
| CHOICE 3 - INTERNATIONAL NEUROBLASTOMA RISK GROUP | |
| To carry staging Hepatoblastoma details for Children, Teenagers and Young Adults (CTYA) cancer. One occurrence of this group is required per Core Site Specific Staging group if selected as the choice. | |
| M/R | Data Set Data Elements |
| M | PRETEXT STAGING SYSTEM STAGE |
| M | PRETEXT STAGING SYSTEM STAGE ANNOTATION FACTORS |
| CHOICE 4 - RETINOBLASTOMA | |
| To carry staging Retinoblastoma details for Children, Teenagers and Young Adults (CTYA) cancer. One occurrence of this group is required per Core Site Specific Staging group if selected as the choice. | |
| M/R | Data Set Data Elements |
| M | INTERNATIONAL STAGING SYSTEM STAGE (RETINOBLASTOMA) |
| TREATMENT: PRINCIPAL TREATMENT CENTRE CHOICE - CHILDREN, TEENAGERS AND YOUNG ADULTS (CTYA) |
|---|
| One of the following Principal Treatment Centre Sections MUST be provided per Core Treatment | |
| CHOICE 1 - CHILDREN | |
| To carry Treatment details for the nominated children's Principal Treatment Centre for Children, Teenagers and Young Adults (CTYA) cancer. One occurrence of this group is required per Core Treatment if selected as the choice. | |
| M/R | Data Set Data Elements |
| M | ORGANISATION IDENTIFIER (CHILDRENS NOMINATED PRINCIPAL TREATMENT CENTRE) Multiple occurrences of this item are permitted |
| CHOICE 2 - TEENAGE YOUNG ADULT (TYA) | |
| To carry Treatment details for the nominated Teenage Young Adult's (TYA) Principal Treatment Centre for Children, Teenagers and Young Adults (CTYA) cancer. One occurrence of this group is required per Core Treatment if selected as the choice. | |
| M/R | Data Set Data Elements |
| M | ORGANISATION IDENTIFIER (TEENAGE YOUNG ADULTS NOMINATED PRINCIPAL TREATMENT CENTRE) Multiple occurrences of this item are permitted |
| TREATMENT: CHILDREN'S CANCER AND LEUKAEMIA GROUP (CCLG) - CHILDREN, TEENAGERS AND YOUNG ADULTS (CTYA) |
|---|
| To carry treatment details for the Children's Cancer and Leukaemia Group (CCLG) guidelines for Children, Teenagers and Young Adults (CTYA) cancer. One occurrence of this group is permitted per Core Treatment. | |
| M/R | Data Set Data Elements |
| R | PATIENT TREATED TO CHILDRENS CANCER AND LEUKAEMIA GROUP GUIDELINES INDICATOR |
| R | CHILDRENS CANCER AND LEUKAEMIA GROUP GUIDELINE NAME |
| LABORATORY RESULTS: NEUROBLASTOMA - CHILDREN, TEENAGERS AND YOUNG ADULTS (CTYA) |
|---|
| To carry Neuroblastoma laboratory result details for Children, Teenagers and Young Adults (CTYA) cancer. One occurrence of this group is permitted per Core Laboratory Results. | |
| M/R | Data Set Data Elements |
| R | URINE VANILLYLMANDELIC ACID CREATININE RATIO |
| RENAL TUMOURS - CHILDREN, TEENAGERS AND YOUNG ADULTS (CTYA) |
|---|
| To carry renal tumour details for Children, Teenagers and Young Adults (CTYA) cancer. One occurrence of this group is permitted. | |
| M/R | Data Set Data Elements |
| R | PATHOLOGICAL RISK CLASSIFICATION CODE (AFTER NEPHRECTOMY) |
| R | PATHOLOGICAL RISK CLASSIFICATION CODE (AFTER PREOPERATIVE CHEMOTHERAPY) |
| RETINOBLASTOMA - CHILDREN, TEENAGERS AND YOUNG ADULTS (CTYA) |
|---|
| To carry Retinoblastoma details for Children, Teenagers and Young Adults (CTYA) cancer. Multiple occurrences of this group are permitted. | |
| M/R | Data Set Data Elements |
| R | RETINOBLASTOMA ASSESSMENT LATERALITY |
| R | INTERNATIONAL CLASSIFICATION FOR INTRAOCULAR RETINOBLASTOMA |
| CHEMOTHERAPY - CHILDREN, TEENAGERS AND YOUNG ADULTS (CTYA) |
|---|
| To carry chemotherapy details for Children, Teenagers and Young Adults (CTYA) cancer. One occurrence of this group is permitted. | |
| M/R | Data Set Data Elements |
| R | CHILDREN TEENAGERS AND YOUNG ADULTS AGE CATEGORY (CONSULTANT PRESCRIBING CHEMOTHERAPY) |
Change to Data Set: Changed Description
Cancer Outcomes and Services Data Set Overview
Due to the rapidly changing situation with Covid-19, the implementation date of the Cancer Outcomes and Services Data Set (COSDS) has been deferred until 1 July 2020.
This will be reviewed and continued to be moved forward 3 months until there is a stable position nationally to resume the upgrade process.
For further information please contact: COSD@phe.gov.uk.
The previous version of the data set can be found at: COSDS Data Sets.
For a "Full Screen" view, click Cancer Outcomes and Services Data Set: Colorectal.
In the "Full Screen" view, to return to the "Data Set" view, click the browser "back" button.
The Mandatory or Required (M/R) column in the COSDS XML Schema indicates the recommendation for the inclusion of data.
- M = Mandatory: this data element is mandatory and the technical process (e.g. submission of the data set, production of output etc.) cannot be completed without this data element being present
- R = Required: NHS business processes cannot be delivered without this data element.
For guidance on downloading the XML Schema, see XML Schema TRUD Download.
For guidance on the XML Schema constraints, see the Cancer Outcomes and Services Data Set XML Schema Constraints.
| DIAGNOSIS - COLORECTAL |
|---|
| To carry diagnostic details for Colorectal cancer. One occurrence of this group is permitted per Core Diagnosis. | |
| M/R | Data Set Data Elements |
| R | SYNCHRONOUS TUMOUR COLON LOCATION (AT DIAGNOSIS) Multiple occurrences of this item are permitted |
| R | TUMOUR HEIGHT ABOVE ANAL VERGE |
| CLINICAL NURSE SPECIALIST - COLORECTAL |
|---|
| To carry details of the Clinical Nurse Specialist. Multiple occurrences of this group are permitted per Core Clinical Nurse Specialist and Risk Factor. | |
| M/R | Data Set Data Elements |
| R | CLINICAL NURSE SPECIALIST TYPE |
Change to Data Set: Changed Description
Cancer Outcomes and Services Data Set Overview
Due to the rapidly changing situation with Covid-19, the implementation date of the Cancer Outcomes and Services Data Set (COSDS) has been deferred until 1 July 2020.This will be reviewed and continued to be moved forward 3 months until there is a stable position nationally to resume the upgrade process.
For further information please contact: COSD@phe.gov.uk.
The previous version of the data set can be found at: COSDS Data Sets.
For a "Full Screen" view, click Cancer Outcomes and Services Data Set: Core.
In the "Full Screen" view, to return to the "Data Set" view, click the browser "back" button.
The Mandatory, Required or Optional (M/R/O) column in the COSDS XML Schema indicates the recommendation for the inclusion of data.
- M = Mandatory: this data element is mandatory and the technical process (e.g. submission of the data set, production of output etc.) cannot be completed without this data element being present
- R = Required: NHS business processes cannot be delivered without this data element
- O = Optional: the inclusion of this data element is optional as required for local purposes.
For guidance on downloading the XML Schema, see XML Schema TRUD Download.
For guidance on the XML Schema constraints, see the Cancer Outcomes and Services Data Set XML Schema Constraints.
| SUBMISSION HEADER |
|---|
| To carry the submission header details. One occurrence of this group is required. | |
| M/R/O | Data Set Data Elements |
| M | COSDS SUBMISSION IDENTIFIER |
| M | ORGANISATION IDENTIFIER (CODE OF SUBMITTING ORGANISATION) |
| M | COSDS SUBMISSION RECORD COUNT |
| M | REPORTING PERIOD START DATE |
| M | REPORTING PERIOD END DATE |
| M | DATE AND TIME DATA SET CREATED |
| RECORD IDENTIFIER |
|---|
| To carry the record identifier details. One occurrence of this group is required. | |
| M/R/O | Data Set Data Elements |
| M | COSDS UNIQUE IDENTIFIER |
| LINKAGE: IDENTIFIER CHOICE - CORE |
|---|
| One of the following Core Linkage Identifier sections MUST be provided | |
| CHOICE 1 - NHS NUMBER | |
| To carry patient identity details for linkage. One occurrence of this group is required if selected as the choice. | |
| M/R/O | Data Set Data Elements |
| M | NHS NUMBER |
| CHOICE 2 - LOCAL PATIENT IDENTIFIER | |
| To carry patient identity details for linkage. One occurrence of this group is required if selected as the choice. | |
| M/R/O | Data Set Data Elements |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| LINKAGE: PATIENT IDENTITY DETAILS - CORE |
|---|
| To carry patient identity details for linkage. One occurrence of this group is required if selected as the choice. | |
| M/R/O | Data Set Data Elements |
| M | NHS NUMBER STATUS INDICATOR CODE |
| M | PERSON BIRTH DATE |
| M | ORGANISATION IDENTIFIER (CODE OF PROVIDER) |
| DIAGNOSTIC: CANCER PATHWAY CHOICE - CORE |
|---|
| One of the following Cancer Pathway sections MUST be provided | |
| CHOICE 1 - PRIMARY CANCER PATHWAY | |
| To carry diagnostic details for linkage. One occurrence of this group is required if selected as the choice. | |
| M/R/O | Data Set Data Elements |
| M | PRIMARY DIAGNOSIS (ICD) |
| M | TUMOUR LATERALITY |
| M | DATE OF PRIMARY CANCER DIAGNOSIS (CLINICALLY AGREED) |
| CHOICE 2 - NON PRIMARY CANCER PATHWAY | |
| To carry diagnostic details for linkage. One occurrence of this group is required if selected as the choice. | |
| M/R/O | Data Set Data Elements |
| M | DATE OF NON PRIMARY CANCER DIAGNOSIS (CLINICALLY AGREED) |
| DIAGNOSTIC: NON PRIMARY CANCER PATHWAY - CORE |
|---|
| One of the following Core Diagnosis sections MUST be provided per Non Primary Cancer Pathway | |
| CHOICE 1 - CANCER RECURRENCE | |
| To carry patient pathway details required to define the Non Primary Cancer Pathway for Cancer Recurrence. One occurrence of this group is required if selected as the choice. | |
| M/R/O | Data Set Data Elements |
| R | PRIMARY DIAGNOSIS (ICD ORIGINAL) |
| M | CANCER METASTATIC DISEASE TYPE Multiple occurrences of this item are permitted |
| M | METASTATIC SITE (AT DIAGNOSIS) Multiple occurrences of this item are permitted |
| R | PALLIATIVE CARE SPECIALIST SEEN INDICATOR (CANCER RECURRENCE) |
| R | RELAPSE METHOD DETECTION TYPE Multiple occurrences of this item are permitted |
| CHOICE 2 - CANCER PROGRESSION | |
| To carry patient pathway details required to define the Non Primary Cancer Pathway for Cancer Progression. One occurrence of this group is required if selected as the choice. | |
| M/R/O | Data Set Data Elements |
| M | CANCER PROGRESSION (ICD ORIGINAL) |
| M | CANCER METASTATIC DISEASE TYPE Multiple occurrences of this item are permitted |
| M | METASTATIC SITE (AT DIAGNOSIS) Multiple occurrences of this item are permitted |
| CHOICE 3 - CANCER TRANSFORMATION | |
| To carry patient pathway details required to define the Non Primary Cancer Pathway for Cancer Transformation. One occurrence of this group is required if selected as the choice. | |
| M/R/O | Data Set Data Elements |
| R | MORPHOLOGY (ICD-O CANCER TRANSFORMATION ORIGINAL) |
| R | MORPHOLOGY (SNOMED CANCER TRANSFORMATION ORIGINAL) |
| DIAGNOSTIC: NON PRIMARY CANCER PATHWAY: TRANSFORMATION CURRENT MORPHOLOGY CHOICE - CORE |
|---|
| At least one of the following MUST be provided per Cancer Transformation | |
| CHOICE 1 - CANCER TRANSFORMATION: CURRENT MORPHOLOGY ICD | |
| To carry patient pathway details required to define the Non Primary Cancer Pathway for Cancer Transformation Current Morphology (ICD). One occurrence of this group is required if selected as the choice. | |
| M/R/O | Data Set Data Elements |
| M | MORPHOLOGY (ICD-O CANCER TRANSFORMATION) |
| CHOICE 2 - CANCER TRANSFORMATION: CURRENT MORPHOLOGY SNOMED | |
| To carry patient pathway details required to define the Non Primary Cancer Pathway for Cancer Transformation Current Morphology (SNOMED). One occurrence of this group is permitted if selected as the choice. | |
| M/R/O | Data Set Data Elements |
| M | MORPHOLOGY (SNOMED CANCER TRANSFORMATION) |
| M | SNOMED VERSION (CANCER TRANSFORMATION) |
| DEMOGRAPHICS - CORE |
|---|
| To carry patient demographic details. One occurrence of this group is permitted. | |
| M/R/O | Data Set Data Elements |
| R | PERSON FAMILY NAME |
| R | PERSON GIVEN NAME |
| R | PATIENT USUAL ADDRESS (AT DIAGNOSIS) - ADDRESS STRUCTURED or PATIENT USUAL ADDRESS (AT DIAGNOSIS) - ADDRESS UNSTRUCTURED |
| R | POSTCODE OF USUAL ADDRESS (AT DIAGNOSIS) |
| R | PERSON STATED GENDER CODE |
| R | PERSON STATED SEXUAL ORIENTATION CODE (AT DIAGNOSIS)` |
| R | GENERAL MEDICAL PRACTITIONER (SPECIFIED) |
| R | GENERAL MEDICAL PRACTICE CODE (PATIENT REGISTRATION) |
| R | PERSON FAMILY NAME (AT BIRTH) |
| R | ETHNIC CATEGORY |
| REFERRALS AND FIRST STAGE OF PATIENT PATHWAY - CORE |
|---|
| To carry patient referral details to the trust that receives the first referral. These details include information relating to the first stage of the Patient Pathway. One occurrence of this group is permitted per Primary Cancer Pathway. | |
| M/R/O | Data Set Data Elements |
| R | SOURCE OF REFERRAL FOR OUT-PATIENTS |
| R | DATE FIRST SEEN |
| M | PROFESSIONAL REGISTRATION ISSUER CODE (CANCER FIRST SEEN) |
| M | PROFESSIONAL REGISTRATION ENTRY IDENTIFIER (CANCER FIRST SEEN) |
| R | ORGANISATION SITE IDENTIFIER (OF PROVIDER FIRST SEEN) |
| R | DATE FIRST SEEN (CANCER SPECIALIST) |
| R | ORGANISATION SITE IDENTIFIER (OF PROVIDER FIRST CANCER SPECIALIST) |
| R | CANCER SYMPTOMS FIRST NOTED DATE |
| NON PRIMARY CANCER PATHWAY: REFERRAL - CORE |
|---|
| To carry non primary cancer pathway details. One occurrence of this group is permitted per Non Primary Cancer Pathway. | |
| M/R/O | Data Set Data Elements |
| R | SOURCE OF REFERRAL FOR OUT-PATIENTS (NON PRIMARY CANCER PATHWAY) |
| R | DATE FIRST SEEN (NON CANCER PRIMARY PATHWAY) |
| R | ORGANISATION SITE IDENTIFIER (OF PROVIDER FIRST SEEN NON PRIMARY CANCER PATHWAY) |
| IMAGING - CORE |
|---|
| To carry imaging details. Multiple occurrences of this group are permitted. | |
| M/R/O | Data Set Data Elements |
| M | ORGANISATION SITE IDENTIFIER (OF IMAGING) |
| M | PROCEDURE DATE (CANCER IMAGING) |
| R | CANCER IMAGING OUTCOME |
| IMAGING: CANCER SITE LOCATION CHOICE - CORE |
|---|
| One of the following Core Imaging data items or sections can be provided per Core Imaging | |
| CHOICE 1 - NICIP | |
| To carry imaging NICIP details. Multiple occurrences of this group are permitted per Core Imaging. | |
| M/R/O | Data Set Data Elements |
| M | IMAGING CODE (NICIP) |
| CHOICE 2 - SNOMED CT | |
| To carry imaging SNOMED CT details. Multiple occurrences of this group are permitted per Core Imaging. | |
| M/R/O | Data Set Data Elements |
| M | IMAGING CODE (SNOMED CT) |
| CHOICE 3 - CANCER SITE IMAGING LOCATION GROUP | |
| To carry imaging details. Multiple occurrences of this group are permitted per Core Imaging. | |
| M/R/O | Data Set Data Elements |
| M | CANCER IMAGING MODALITY |
| R | IMAGING ANATOMICAL SITE |
| R | ANATOMICAL SIDE (IMAGING) |
| To carry imaging details. Multiple occurrences of this group are permitted. | |
| M/R/O | Data Set Data Elements |
| R | IMAGING REPORT TEXT |
| R | LESION SIZE (RADIOLOGICAL) |
| DIAGNOSTIC PROCEDURES - CORE |
|---|
| To carry diagnostic procedure details. Multiple occurrences of this group are permitted. | |
| M/R/O | Data Set Data Elements |
| M | ORGANISATION SITE IDENTIFIER (OF DIAGNOSTIC PROCEDURE) |
| M | PROCEDURE DATE (DIAGNOSTIC PROCEDURE) |
| DIAGNOSTIC PROCEDURES: CHOICE - CORE |
|---|
| One of the following Core Diagnostic procedures data items MUST be provided per Core Diagnostic Procedures | |
| CHOICE 1 - OPCS | |
| To carry OPCS diagnostic procedure details. Multiple occurrences of this group are permitted per Core Diagnostic Procedures. | |
| M/R/O | Data Set Data Elements |
| M | DIAGNOSTIC PROCEDURE (OPCS) |
| CHOICE 2 - SNOMED CT | |
| To carry SNOMED CT diagnostic procedure details. Multiple occurrences of this group are permitted per Core Diagnostic Procedures. | |
| M/R/O | Data Set Data Elements |
| M | DIAGNOSTIC PROCEDURE (SNOMED CT) |
| DIAGNOSTIC PROCEDURES: SENTINEL NODE BIOPSY - CORE |
|---|
| To carry diagnostic details for Sentinel Node Biopsy. One occurrence of this group is permitted per Core Diagnostic Procedures. | |
| M/R/O | Data Set Data Elements |
| R | SENTINEL LYMPH NODE BIOPSY OUTCOME |
| DIAGNOSIS - CORE |
|---|
| To carry diagnostic details for the Primary Diagnosis. One occurrence of this group is permitted per Primary Cancer Pathway. | |
| M/R/O | Data Set Data Elements |
| R | ORGANISATION SITE IDENTIFIER (OF DIAGNOSIS) |
| R | BASIS OF DIAGNOSIS (CANCER) |
| R | MORPHOLOGY (ICD-O DIAGNOSIS) |
| M | MORPHOLOGY (SNOMED DIAGNOSIS) |
| M | SNOMED VERSION (DIAGNOSIS) |
| R | TOPOGRAPHY (ICD-O) |
| R | GRADE OF DIFFERENTIATION (AT DIAGNOSIS) |
| R | PERFORMANCE STATUS (ADULT) |
| R | DIAGNOSIS (SNOMED CT) |
| M | CANCER METASTATIC DISEASE TYPE Multiple occurrences of this item are permitted |
| M | METASTATIC SITE (AT DIAGNOSIS) Multiple occurrences of this item are permitted |
| DIAGNOSIS: ADDITIONAL ITEMS - CORE |
|---|
| To carry additional diagnostic details. One occurrence of this group is permitted per Core Diagnosis. | |
| M/R/O | Data Set Data Elements |
| R | PRIMARY DIAGNOSIS (CANCER COMMENT) |
| R | SECONDARY DIAGNOSIS (ICD) Multiple occurrences of this item are permitted |
| R | SECONDARY DIAGNOSIS (CANCER COMMENT) |
| R | FAMILIAL CANCER SYNDROME INDICATOR |
| R | FAMILIAL CANCER SYNDROME COMMENT |
| DIAGNOSIS: CANCER PROGRESSION - CORE |
|---|
| To carry additional diagnostic details for Cancer Progression. May be multiple occurrences per Core Diagnosis. | |
| M/R/O | Data Set Data Elements |
| M | CANCER METASTATIC DISEASE TYPE Multiple occurrences of this item are permitted |
| M | METASTATIC SITE (AT DIAGNOSIS) Multiple occurrences of this item are permitted |
| M | CANCER PROGRESSION AGREED DATE (PRIMARY CANCER PATHWAY) |
| DIAGNOSIS: CANCER TRANSFORMATION - CORE |
|---|
| To carry additional diagnostic details for Cancer Transformation. May be multiple occurrences per Core Diagnosis. | |
| M/R/O | Data Set Data Elements |
| M | CANCER TRANSFORMATION AGREED DATE (PRIMARY CANCER PATHWAY) |
| DIAGNOSIS: CANCER TRANSFORMATION CHOICE - CORE |
|---|
| At least one of the following MUST be provided per Cancer Transformation | |
| CHOICE 1 - ICD | |
| To carry ICD additional diagnostic details for Cancer Transformation. One occurrence of this group is required per Core Diagnosis Cancer Transformation. | |
| M/R/O | Data Set Data Elements |
| M | MORPHOLOGY (ICD-O CANCER TRANSFORMATION) |
| CHOICE 2 - SNOMED | |
| To carry additional diagnostic details for Cancer Transformation. One occurrence of this group is required per Core Diagnosis Cancer Transformation. | |
| M/R/O | Data Set Data Elements |
| M | MORPHOLOGY (SNOMED CANCER TRANSFORMATION) |
| M | SNOMED VERSION (CANCER TRANSFORMATION) |
| DIAGNOSIS: BANKED TISSUE - CORE |
|---|
| To carry Banked Tissue details. One occurrence of this group is permitted per Core Diagnosis. | |
| M/R/O | Data Set Data Elements |
| R | PATIENT CONSENT FOR TISSUE BANKED AT DIAGNOSIS INDICATION CODE |
| R | TISSUE TYPE BANKED AT DIAGNOSIS (CANCER) Multiple occurrences of this item are permitted |
| PERSON OBSERVATION - CORE |
|---|
| To carry person observation details. Multiple occurrences of this group are permitted. | |
| M/R/O | Data Set Data Elements |
| R | PERSON HEIGHT IN METRES |
| R | PERSON WEIGHT |
| R | BODY MASS INDEX |
| M | OBSERVATION DATE |
| CLINICAL NURSE SPECIALIST AND RISK FACTOR ASSESSMENT - CORE |
|---|
| To carry Clinical Nurse Specialist and risk factor assessment details. One occurrence of this group is permitted. | |
| M/R/O | Data Set Data Elements |
| R | CLINICAL NURSE SPECIALIST INDICATION CODE |
| R | SMOKING STATUS (CANCER) |
| R | TOBACCO SMOKING CESSATION TREATMENT INDICATION CODE |
| R | ALCOHOL HISTORY (CANCER IN LAST THREE MONTHS) |
| R | ALCOHOL HISTORY (CANCER BEFORE LAST THREE MONTHS) |
| R | PATIENT DIAGNOSIS INDICATOR (DIABETES) |
| R | MENOPAUSAL STATUS (AT DIAGNOSIS) |
| R | PHYSICAL ACTIVITY VITAL SIGN LEVEL (CURRENT) |
| CLINICAL NURSE SPECIALIST: HOLISTIC NEEDS ASSESSMENT (HNA) - CORE |
|---|
| To carry details of Clinical Nurse Specialist, Holistic Needs Assessments. Multiple occurrences of this group are permitted. | |
| M/R/O | Data Set Data Elements |
| R | OFFER STATUS (HOLISTIC NEEDS ASSESSMENT) |
| R | HOLISTIC NEEDS ASSESSMENT COMPLETED DATE |
| R | HOLISTIC NEEDS ASSESSMENT POINT OF PATHWAY (CANCER) |
| R | STAFF ROLE CARRYING OUT HOLISTIC NEEDS ASSESSMENT |
| CLINICAL NURSE SPECIALIST: PERSONALISED CARE AND SUPPORT PLANNING - CORE |
|---|
| To carry details of Clinical Nurse Specialist, Personalised Care and Supportive Planning Assessments. Multiple occurrences of this group are permitted. | |
| M/R/O | Data Set Data Elements |
| R | OFFER STATUS (PERSONALISED CARE AND SUPPORT PLANNING) |
| R | PERSONALISED CARE AND SUPPORT PLANNING COMPLETED DATE |
| R | PERSONALISED CARE AND SUPPORT PLANNING POINT OF CANCER PATHWAY |
| R | STAFF ROLE CARRYING OUT PERSONALISED CARE AND SUPPORT PLANNING |
| MULTIDISCIPLINARY TEAM MEETINGS CHOICE - CORE |
|---|
| One of the following Core Multidisciplinary Team Meeting data items MUST be provided per Core Multidisciplinary Team Meeting | |
| CHOICE 1 - NOT DISCUSSED | |
| To carry details of all multidisciplinary team meetings where the patient was not discussed. One occurrence of this group is required per Core Multidisciplinary Team Meetings if selected as the choice. | |
| M/R/O | Data Set Data Elements |
| M | MULTIDISCIPLINARY TEAM MEETING CANCER CARE PLAN NOT DISCUSSED INDICATION CODE |
| CHOICE 2 - DISCUSSED | |
| To carry details of all multidisciplinary team meetings where the patient was discussed. One occurrence of this group is required per Core Multidisciplinary Team Meetings if selected as the choice. | |
| M/R/O | Data Set Data Elements |
| M | MULTIDISCIPLINARY TEAM MEETING CANCER CARE PLAN DISCUSSION TYPE |
| M | MULTIDISCIPLINARY TEAM MEETING DATE (CANCER) |
| M | ORGANISATION SITE IDENTIFIER (OF MULTIDISCIPLINARY TEAM MEETING) |
| M | MULTIDISCIPLINARY TEAM MEETING TYPE (CANCER) |
| R | MULTIDISCIPLINARY TEAM MEETING TYPE COMMENT (CANCER) |
| CANCER CARE PLAN - CORE |
|---|
| To carry cancer care plan details. One occurrence of this group is permitted per Primary Cancer Pathway. | |
| M/R/O | Data Set Data Elements |
| R | MULTIDISCIPLINARY TEAM DISCUSSION DATE (CANCER) |
| M | PROFESSIONAL REGISTRATION ISSUER CODE (MULTIDISCIPLINARY TEAM LEAD) |
| M | PROFESSIONAL REGISTRATION ENTRY IDENTIFIER (MULTIDISCIPLINARY TEAM LEAD) |
| R | CANCER CARE PLAN INTENT |
| R | PLANNED CANCER TREATMENT TYPE Multiple occurrences of this item are permitted |
| R | NO CANCER TREATMENT REASON |
| O | ADULT COMORBIDITY EVALUATION - 27 SCORE |
| MOLECULAR AND BIOMARKERS - GERMLINE TESTING FOR CANCER PREDISPOSITION - CORE |
|---|
| To carry Molecular and Biomarkers (Germline Testing for Cancer Predisposition) details for a patient. Multiple occurrences of this group are permitted. | |
| M/R/O | Data Set Data Elements |
| R | OFFER STATUS (GERMLINE GENETIC TEST) |
| R | GERMLINE GENETIC TEST TYPE OFFERED Multiple occurrences of this item are permitted |
| R | OTHER GERMLINE GENETIC TEST TYPE OFFERED COMMENT Multiple occurrences of this item are permitted |
| R | ACTIVITY OFFER DATE (GERMLINE GENETIC TEST) |
| R | ORGANISATION IDENTIFIER (REPORTING LABORATORY) |
| R | OFFER STATUS (REFERRAL TO REGIONAL CLINICAL GENETICS SERVICE) |
| MOLECULAR AND BIOMARKERS - SOMATIC TESTING FOR TARGETED THERAPY AND PERSONALISED MEDICINE - CORE |
|---|
| To carry Molecular and Biomarkers (Somatic Testing for Targeted Therapy and Personalised Medicine) details for a patient, where these have been performed by the clinical teams. Multiple occurrences of this group are permitted. | |
| M/R/O | Data Set Data Elements |
| R | GENE OR STRATIFICATION BIOMARKER TYPE ANALYSED Multiple occurrences of this item are permitted |
| R | OTHER GENE OR STRATIFICATION BIOMARKER TYPE ANALYSED COMMENT Multiple occurrences of this item are permitted |
| R | GENE OR STRATIFICATION BIOMARKER REPORTED DATE |
| R | ORGANISATION IDENTIFIER (REPORTING LABORATORY) |
| CLINICAL TRIALS - CORE |
|---|
| To carry details for a patient who is eligible for a cancer clinical trial. Multiple occurrences of this group are permitted. | |
| M/R/O | Data Set Data Elements |
| R | PATIENT TRIAL STATUS (CANCER) |
| R | CLINICAL TRIAL DECISION DATE |
| R | CLINICAL TRIAL START DATE |
| R | CANCER CLINICAL TRIAL TREATMENT TYPE |
| STAGING - CORE |
|---|
| To carry the staging details at the time that the first cancer care plan is agreed. One occurrence of this group is permitted per Primary Cancer Pathway. | |
| M/R/O | Data Set Data Elements |
| R | T CATEGORY (FINAL PRETREATMENT) |
| R | N CATEGORY (FINAL PRETREATMENT) |
| R | M CATEGORY (FINAL PRETREATMENT) |
| R | TNM STAGE GROUPING (FINAL PRETREATMENT) |
| R | ORGANISATION SITE IDENTIFIER (OF TNM STAGE GROUPING FINAL PRETREATMENT) |
| R | TNM STAGE GROUPING DATE (FINAL PRETREATMENT) |
| R | T CATEGORY (INTEGRATED STAGE) |
| R | N CATEGORY (INTEGRATED STAGE) |
| R | M CATEGORY (INTEGRATED STAGE) |
| R | TNM STAGE GROUPING (INTEGRATED) |
| R | ORGANISATION SITE IDENTIFIER (OF TNM STAGE GROUPING INTEGRATED) |
| R | TNM STAGE GROUPING DATE (INTEGRATED) |
| M | TNM CODING EDITION |
| M | TNM VERSION NUMBER (STAGING) |
| SITE SPECIFIC STAGING - CORE |
|---|
| To carry the site specific cancer staging details. These fields are only required where there is a site specific stage recorded for a patient and will not be applicable to every cancer. Multiple occurrences of this group are permitted. | |
| M/R/O | Data Set Data Elements |
| M | ORGANISATION SITE IDENTIFIER (OF CANCER SITE SPECIFIC STAGE) |
| M | STAGE DATE (CANCER SITE SPECIFIC STAGE) |
| TREATMENT - CORE |
|---|
| To carry the cancer treatment details. Multiple occurrences of this group are permitted. | |
| M/R/O | Data Set Data Elements |
| R | ADJUNCTIVE THERAPY TYPE |
| R | CANCER TREATMENT INTENT |
| M | TREATMENT START DATE (CANCER) |
| M | CANCER TREATMENT MODALITY (REGISTRATION) |
| M | ORGANISATION SITE IDENTIFIER (OF PROVIDER CANCER TREATMENT START DATE) |
| M | PROFESSIONAL REGISTRATION ISSUER CODE (TREATMENT) |
| M | PROFESSIONAL REGISTRATION ENTRY IDENTIFIER (TREATMENT) |
| O | CANCER END OF TREATMENT SUMMARY PLAN COMPLETION DATE Multiple occurrences of this item are permitted |
| R | DISCHARGE DATE (HOSPITAL PROVIDER SPELL) |
| R | DISCHARGE DESTINATION CODE (HOSPITAL PROVIDER SPELL) |
| TREATMENT: SURGERY - CORE |
|---|
| To carry the surgery details. One occurrence of this group is permitted per Core Treatment. | |
| M/R/O | Data Set Data Elements |
| M | PROCEDURE DATE |
| R | CANCER SURGICAL ADMISSION TYPE |
| M | PROFESSIONAL REGISTRATION ISSUER CODE (RESPONSIBLE SURGEON) Multiple occurrences of this item are permitted |
| M | PROFESSIONAL REGISTRATION ENTRY IDENTIFIER (RESPONSIBLE SURGEON) Multiple occurrences of this item are permitted |
| R | PRIMARY PROCEDURE (OPCS) |
| R | PRIMARY PROCEDURE (SNOMED CT) |
| R | PROCEDURE (OPCS) Multiple occurrences of this item are permitted |
| R | PROCEDURE (SNOMED CT) Multiple occurrences of this item are permitted |
| R | ADDITIONAL UNPLANNED PROCEDURE REQUIRED INDICATOR |
| R | ASA PHYSICAL STATUS CLASSIFICATION SYSTEM CODE |
| R | SURGICAL ACCESS TYPE |
| TREATMENT: STEM CELL TRANSPLANTATION - CORE |
|---|
| To carry surgery Stem Cell Transplantation details. One occurrence of this group is permitted per Core Treatment. | |
| M/R/O | Data Set Data Elements |
| R | STEM CELL INFUSION SOURCE CODE |
| R | STEM CELL INFUSION DONOR TYPE |
| R | STEM CELL TRANSPLANT CONDITIONING REGIMEN |
| ACUTE ONCOLOGY - CORE |
|---|
| To carry Acute Oncology details. Multiple occurrences of this group are permitted. | |
| M/R/O | Data Set Data Elements |
| R | ACUTE ONCOLOGY ASSESSMENT COMPLETED DATE |
| R | ORGANISATION SITE IDENTIFIER (OF ACUTE ONCOLOGY ASSESSMENT) |
| R | ACUTE ONCOLOGY ASSESSMENT LOCATION |
| R | ACUTE ONCOLOGY ASSESSMENT PATIENT PRESENTATION TYPE Multiple occurrences of this item are permitted |
| R | ACUTE ONCOLOGY EPISODE OUTCOME |
| LABORATORY RESULTS - CORE |
|---|
| To carry laboratory result details. Multiple occurrences of this group are permitted. | |
| M/R/O | Data Set Data Elements |
| M | LABORATORY RESULT AUTHORISED DATE |
| M | ORGANISATION IDENTIFIER (OF LABORATORY RESULT) |
| LABORATORY RESULTS: GENERAL - CORE |
|---|
| To carry general laboratory result details. One occurrence of this group is permitted per Core Laboratory Results. | |
| M/R/O | Data Set Data Elements |
| R | LACTATE DEHYDROGENASE LEVEL (PEAK AT DIAGNOSIS) |
| R | BETA HUMAN CHORIONIC GONADOTROPIN (MAXIMUM AT DIAGNOSIS) |
| R | ALPHA FETOPROTEIN (MAXIMUM AT DIAGNOSIS) |
Change to Data Set: Changed Description
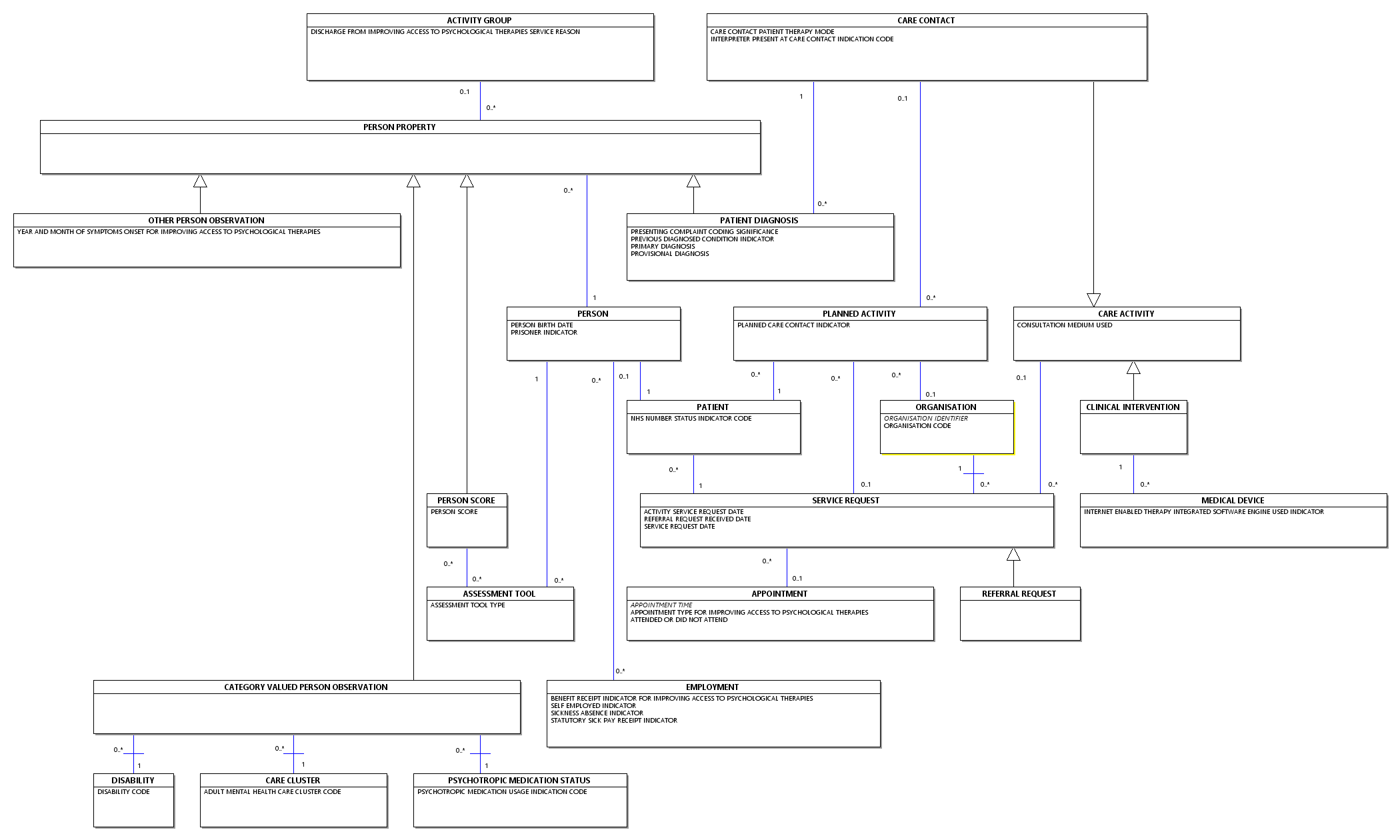
Cancer Outcomes and Services Data Set Overview
Due to the rapidly changing situation with Covid-19, the implementation date of the Cancer Outcomes and Services Data Set (COSDS) has been deferred until 1 July 2020.
This will be reviewed and continued to be moved forward 3 months until there is a stable position nationally to resume the upgrade process.
For further information please contact: COSD@phe.gov.uk.
The previous version of the data set can be found at: COSDS Data Sets.
For a "Full Screen" view, click Cancer Outcomes and Services Data Set: Gynaecological.
In the "Full Screen" view, to return to the "Data Set" view, click the browser "back" button.
The Mandatory or Required (M/R) column in the COSDS XML Schema indicates the recommendation for the inclusion of data.
- M = Mandatory: this data element is mandatory and the technical process (e.g. submission of the data set, production of output etc.) cannot be completed without this data element being present
- R = Required: NHS business processes cannot be delivered without this data element.
For guidance on downloading the XML Schema, see XML Schema TRUD Download.
For guidance on the XML Schema constraints, see the Cancer Outcomes and Services Data Set XML Schema Constraints.
| SITE SPECIFIC STAGING - GYNAECOLOGICAL |
|---|
| To carry staging details for Gynaecological cancer. One occurrence of this group is permitted per Core Site Specific Staging. | |
| M/R | Data Set Data Elements |
| M | FINAL FIGO STAGE |
| TREATMENT: SURGERY - GYNAECOLOGICAL |
|---|
| To carry surgery details for Gynaecological cancer. One occurrence of this group is permitted per Core Treatment Surgery. | |
| M/R | Data Set Data Elements |
| R | CARE PROFESSIONAL SENIOR OPERATING SURGEON GRADE (CANCER) |
| R | RESIDUAL DISEASE SIZE (GYNAECOLOGICAL CANCER) |
Change to Data Set: Changed Description
Cancer Outcomes and Services Data Set Overview
Due to the rapidly changing situation with Covid-19, the implementation date of the Cancer Outcomes and Services Data Set (COSDS) has been deferred until 1 July 2020.
This will be reviewed and continued to be moved forward 3 months until there is a stable position nationally to resume the upgrade process.
For further information please contact: COSD@phe.gov.uk.
The previous version of the data set can be found at: COSDS Data Sets.
For a "Full Screen" view, click Cancer Outcomes and Services Data Set: Haematological.
In the "Full Screen" view, to return to the "Data Set" view, click the browser "back" button.
The Mandatory or Required (M/R) column in the COSDS XML Schema indicates the recommendation for the inclusion of data.
- M = Mandatory: this data element is mandatory and the technical process (e.g. submission of the data set, production of output etc.) cannot be completed without this data element being present
- R = Required: NHS business processes cannot be delivered without this data element.
For guidance on downloading the XML Schema, see XML Schema TRUD Download.
For guidance on the XML Schema constraints, see the Cancer Outcomes and Services Data Set XML Schema Constraints.
| CANCER CARE PLAN CHOICE - HAEMATOLOGICAL |
|---|
| One of the following Cancer Care Plan Sections MUST be provided per Core Cancer Care Plan | |
| CHOICE 1 - CHRONIC MYELOID LEUKAEMIA (CML) | |
| To carry cancer care plan details specific to Chronic Myeloid Leukaemia (CML) for Haematological cancer. One occurrence of this group is required per Core Cancer Care Plan if selected as the choice. | |
| M/R | Data Set Data Elements |
| M | CHRONIC MYELOID LEUKAEMIA INDEX SCORE (SOKAL) |
| CHOICE 2 - MYELODYSPLASIA | |
| To carry cancer care plan details specific to Myelodysplasia for Haematological cancer. One occurrence of this group is required per Core Cancer Care Plan if selected as the choice. | |
| M/R | Data Set Data Elements |
| M | REVISED INTERNATIONAL PROGNOSTIC SCORING SYSTEM SCORE |
| CHOICE 3 - CHRONIC LYMPHOID LEUKAEMIA (CLL) | |
| To carry cancer care plan details specific to Chronic Lymphoid Leukaemia (CLL) for Haematological cancer. One occurrence of this group is required per Core Cancer Care Plan if selected as the choice. | |
| M/R | Data Set Data Elements |
| M | SPLENOMEGALY INDICATOR |
| CHOICE 4 - FOLLICULAR LYMPHOMA | |
| To carry cancer care plan details specific to Follicular Lymphoma for Haematological cancer. One occurrence of this group is required per Core Cancer Care Plan if selected as the choice. | |
| M/R | Data Set Data Elements |
| R | NUMBER OF ABNORMAL NODAL AREAS |
| R | FOLLICULAR LYMPHOMA INTERNATIONAL PROGNOSTIC INDEX 2 SCORE |
| CHOICE 5 - DIFFUSE LARGE B-CELL LYMPHOMA (DLBCL) | |
| To carry cancer care plan details specific to Diffuse Large B-Cell Lymphoma (DLBCL) for Haematological cancer. One occurrence of this group is required per Core Cancer Care Plan if selected as the choice. | |
| M/R | Data Set Data Elements |
| R | NUMBER OF ABNORMAL NODAL AREAS |
| R | PRIMARY EXTRANODAL CANCER SITE |
| R | NUMBER OF EXTRANODAL SITES CODE |
| R | REVISED INTERNATIONAL PROGNOSTIC INDEX SCORE |
| CHOICE 6 - HODGKIN LYMPHOMA | |
| To carry cancer care plan details specific to Hodgkin Lymphoma for Haematological cancer. One occurrence of this group is required per Core Cancer Care Plan if selected as the choice. | |
| M/R | Data Set Data Elements |
| R | NUMBER OF ABNORMAL NODAL AREAS |
| R | PRIMARY EXTRANODAL CANCER SITE |
| R | HASENCLEVER INDEX SCORE |
| CHOICE 7 - ACUTE LYMPHOBLASTIC LEUKAEMIA (ALL) | |
| To carry cancer care plan details specific to Acute Lymphoblastic Leukaemia (ALL) for Haematological cancer. One occurrence of this group is required per Core Cancer Care Plan if selected as the choice. | |
| M/R | Data Set Data Elements |
| M | EXTRAMEDULLARY DISEASE SITE Multiple occurrences of this item are permitted |
| SITE SPECIFIC STAGING - HAEMATOLOGICAL |
|---|
| One of the following Site Specific Staging Sections MUST be provided per Core Site Specific Staging | |
| CHOICE 1 - ANN ARBOR | |
| To carry Ann Arbor site specific staging details for various haematological diseases for Haematological cancer. One occurrence of this group is required per Core Site Specific Staging if selected as the choice. | |
| M/R | Data Set Data Elements |
| M | ANN ARBOR STAGE |
| CHOICE 2 - CHRONIC LYMPHOID LEUKAEMIA (CLL) | |
| To carry Chronic Lymphoid Leukaemia (CLL) staging details for Haematological cancer. One occurrence of this group is required per Core Site Specific Staging if selected as the choice. | |
| M/R | Data Set Data Elements |
| M | BINET STAGE |
| CHOICE 3 - MYELOMA | |
| To carry Myeloma staging details for Haematological cancer. One occurrence of this group is permitted per Core Site Specific Staging if selected as the choice. | |
| M/R | Data Set Data Elements |
| M | REVISED INTERNATIONAL STAGING SYSTEM STAGE FOR MULTIPLE MYELOMA |
| CHOICE 4 - NON HODGKIN LYMPHOMA (NHL) | |
| To carry Non Hodgkin Lymphoma (NHL) staging details for Haematological cancer. One occurrence of this group is required per Core Site Specific Staging if selected as the choice. | |
| M/R | Data Set Data Elements |
| M | MURPHY ST JUDE STAGE |
| ANN ARBOR: EXTENSIONS - HAEMATOLOGICAL |
|---|
| To carry supporting Ann Arbor staging details for various haematological diseases as specified. One occurrence of this group is permitted. | |
| M/R | Data Set Data Elements |
| R | ANN ARBOR SYMPTOMS INDICATION CODE |
| R | ANN ARBOR EXTRANODALITY INDICATION CODE |
| R | ANN ARBOR BULKY DISEASE INDICATION CODE |
| R | ANN ARBOR SPLENIC INDICATION CODE |
| LABORATORY RESULTS CHOICE - HAEMATOLOGICAL |
|---|
| One occurrence of this group is required per Core Laboratory Results One of the following Laboratory Results MUST be provided per Core Laboratory Results | |
| CHOICE 1 - VARIOUS HAEMATOLOGICAL DISEASES | |
| To carry laboratory results for various haematological diseases for Haematological cancer. One occurrence of this group is required per Core Laboratory Results if selected as the choice. | |
| M/R | Data Set Data Elements |
| R | EUROPEAN LEUKAEMIA NET GENETIC RISK CODE |
| R | WHITE BLOOD CELL COUNT (HIGHEST PRETREATMENT) |
| CHOICE 2 - VARIOUS HAEMATOLOGICAL DISEASES: CHILDREN, TEENAGERS AND YOUNG ADULTS (CTYA). | |
| To carry laboratory results for various haematological diseases for Children Teenagers and Young Adults (CTYA) Haematological cancer. One occurrence of this group is required per Core Laboratory Results if selected as the choice. | |
| M/R | Data Set Data Elements |
| R | BONE MARROW BLAST CELLS PERCENTAGE |
| R | CYTOGENETIC FINDINGS COMMENT |
| CHOICE 3 - PAEDIATRIC MYELODYSPLASIA | |
| To carry Paediatric Myelodysplasia laboratory result details for Haematological cancer. One occurrence of this group is required per Core Laboratory Results if selected as the choice. | |
| M/R | Data Set Data Elements |
| R | CELLULARITY PERCENTAGE |
| R | DIEPOXYBUTANE TEST RESULT |
| R | DYSPLASTIC HAEMOPOIESIS TYPE |
| CHOICE 4 - ACUTE LYMPHOBLASTIC LEUKAEMIA (ALL) - RESPONSE | |
| To carry Acute Lymphoblastic Leukaemia (ALL) Response laboratory result details for Haematological cancer. One occurrence of this group is required per Core Laboratory Results if selected as the choice. | |
| M/R | Data Set Data Elements |
| M | LEUKAEMIC CELLS PRESENT POST MINIMAL RESIDUAL DISEASE INDUCTION PERCENTAGE |
| DIAGNOSIS CHOICE - HAEMATOLOGICAL |
|---|
| One of the following can be provided per Core Diagnosis | |
| CHOICE 1 - MIXED PHENOTYPE ACUTE LEUKAEMIA (MPAL) | |
| To carry diagnostic Mixed Phenotype Acute Leukaemia (MPAL) details for Haematological cancer. One occurrence of this group is required per Core Diagnosis if selected as the choice. | |
| M/R | Data Set Data Elements |
| R | MIXED PHENOTYPE ACUTE LEUKAEMIA SYMPTOMS (AT DIAGNOSIS) Multiple occurrences of this item are permitted |
| R | EUROPEAN GROUP FOR THE IMMUNOLOGICAL CLASSIFICATION OF LEUKAEMIA SCORING SYSTEM SCORE |
| CHOICE 2 - ACUTE MYELOID LEUKAEMIA (AML) | |
| To carry diagnostic Acute Myeloid Leukaemia (AML) details for Haematological cancer. One occurrence of this group is required per Core Diagnosis if selected as the choice. | |
| M/R | Data Set Data Elements |
| R | FRENCH AMERICAN BRITISH CLASSIFICATION (ACUTE MYELOID LEUKAEMIA) |
| R | CYTOGENETIC RISK GROUP (PAEDIATRIC MOLECULAR GENETIC ABNORMALITIES) |
| R | ACUTE MYELOID LEUKAEMIA RISK FACTORS (AT DIAGNOSIS) |
| CHOICE 3 - PAEDIATRIC MYELODYSPLASIA | |
| To carry diagnostic Paediatric Myelodysplasia details for Haematological cancer. One occurrence of this group is required per Core Diagnosis if selected as the choice. | |
| M/R | Data Set Data Elements |
| R | PAEDIATRIC MYELODYSPLASIA CLINICAL FINDINGS (AT DIAGNOSIS) Multiple occurrences of this item are permitted |
| R | UNDERLYING DISEASE ASSOCIATED WITH MYELODYSPLASIA (AT DIAGNOSIS) Multiple occurrences of this item are permitted |
| R | CONGENITAL ANOMALIES COMMENT |
| R | OTHER MYELODYSPLASIA SYMPTOMS AT DIAGNOSIS Multiple occurrences of this item are permitted |
| ACUTE LEUKAEMIAS - HAEMATOLOGICAL |
|---|
| To carry treatment details for Acute Leukaemias for Haematological cancer. One occurrence of this group is permitted per Core Treatment. | |
| M/R | Data Set Data Elements |
| R | PRIMARY INDUCTION CHEMOTHERAPY FAILURE INDICATOR |
| MOLECULAR AND BIOMARKERS - SOMATIC TESTING FOR TARGETED THERAPY AND PERSONALISED THERAPY: NON HODGKIN LYMPHOMA - HAEMATOLOGICAL |
|---|
| To carry Molecular and Biomarkers Result details for Non Hodgkin Lymphoma (NML) for Haematological cancer. One occurrence of this group is permitted per Core Molecular and Biomarkers - Somatic Testing for Targeted Therapy and Personalised Medicine. | |
| M/R | Data Set Data Elements |
| M | ALK GENE FUSION STATUS (ANAPLASTIC LARGE CELL LYMPHOMA) |
Change to Data Set: Changed Description
Cancer Outcomes and Services Data Set Overview
For a "Full Screen" view, click Cancer Outcomes and Services Data Set: Head and Neck.Due to the rapidly changing situation with Covid-19, the implementation date of the Cancer Outcomes and Services Data Set (COSDS) has been deferred until 1 July 2020.
This will be reviewed and continued to be moved forward 3 months until there is a stable position nationally to resume the upgrade process.
For further information please contact: COSD@phe.gov.uk.
The previous version of the data set can be found at: COSDS Data Sets.
For a "Full Screen" view, click Cancer Outcomes and Services Data Set: Head and Neck.
In the "Full Screen" view, to return to the "Data Set" view, click the browser "back" button.
The Mandatory or Required (M/R) column in the COSDS XML Schema indicates the recommendation for the inclusion of data.
- M = Mandatory: this data element is mandatory and the technical process (e.g. submission of the data set, production of output etc.) cannot be completed without this data element being present
- R = Required: NHS business processes cannot be delivered without this data element.
For guidance on downloading the XML Schema, see XML Schema TRUD Download.
For guidance on the XML Schema constraints, see the Cancer Outcomes and Services Data Set XML Schema Constraints.
| TREATMENT: SURGERY - HEAD AND NECK |
|---|
| To carry surgery details for Head and Neck cancer. One occurrence of this group is permitted per Core Treatment Surgery. | |
| M/R | Data Set Data Elements |
| R | SURGICAL ACCESS TYPE (HEAD AND NECK CANCER) |
| R | OTHER SURGICAL ACCESS TYPE (HEAD AND NECK CANCER) |
| PRE TREATMENT ASSESSMENT - HEAD AND NECK |
|---|
| To carry pre treatment assessment details for Head and Neck cancer. One occurrence of this group is permitted. | |
| M/R | Data Set Data Elements |
| R | CANCER DENTAL ASSESSMENT DATE |
| R | CARE CONTACT DATE (DIETITIAN INITIAL) |
| R | CARE CONTACT DATE (SPEECH AND LANGUAGE THERAPIST INITIAL) |
| POST TREATMENT ASSESSMENT - HEAD AND NECK |
|---|
| To carry post treatment assessment details for Head and Neck cancer. Multiple occurrences of this group are permitted. | |
| M/R | Data Set Data Elements |
| R | CLINICAL STATUS ASSESSMENT DATE (CANCER) |
| R | PRIMARY TUMOUR STATUS |
| R | NODAL STATUS |
| R | METASTATIC STATUS |
| R | SPEECH AND LANGUAGE ASSESSMENT DATE |
Change to Data Set: Changed Description
Cancer Outcomes and Services Data Set Overview
Due to the rapidly changing situation with Covid-19, the implementation date of the Cancer Outcomes and Services Data Set (COSDS) has been deferred until 1 July 2020.
This will be reviewed and continued to be moved forward 3 months until there is a stable position nationally to resume the upgrade process.
For further information please contact: COSD@phe.gov.uk.
The previous version of the data set can be found at: COSDS Data Sets.
For a "Full Screen" view, click Cancer Outcomes and Services Data Set: Liver.
In the "Full Screen" view, to return to the "Data Set" view, click the browser "back" button.
The Mandatory or Required (M/R) column in the COSDS XML Schema indicates the recommendation for the inclusion of data.
- M = Mandatory: this data element is mandatory and the technical process (e.g. submission of the data set, production of output etc.) cannot be completed without this data element being present
- R = Required: NHS business processes cannot be delivered without this data element.
For guidance on downloading the XML Schema, see XML Schema TRUD Download.
For guidance on the XML Schema constraints, see the Cancer Outcomes and Services Data Set XML Schema Constraints.
| DIAGNOSIS - LIVER |
|---|
| To carry diagnostic details for Liver cancer. One occurrence of this group is permitted per Core Diagnosis. | |
| M/R | Data Set Data Elements |
| R | LIVER CANCER SURVEILLANCE SCAN INDICATOR |
| R | LIVER CIRRHOSIS TYPE |
| R | LIVER CIRRHOSIS CAUSE TYPE Multiple occurrences of this item are permitted |
| DIAGNOSIS: CHOLANGIOCARCINOMA - LIVER |
|---|
| To carry diagnostic details for Cholangiocarcinoma for Liver cancer. One occurrence of this group is permitted per Core Diagnosis. | |
| M/R | Data Set Data Elements |
| M | CHOLANGIOCARCINOMA PRESENCE CATEGORY |
| SITE SPECIFIC STAGING - LIVER |
|---|
| To carry site specific staging details for Liver cancer. One occurrence of this group is permitted per Core Site Specific Staging. | |
| M/R | Data Set Data Elements |
| M | BARCELONA CLINIC LIVER CANCER STAGE |
| TREATMENT AND PROGNOSTIC INDICATORS - LIVER |
|---|
| To carry treatment and prognostic details for Liver cancer. One occurrence of this group is permitted. | |
| M/R | Data Set Data Elements |
| R | PORTAL VEIN INVASION INDICATION CODE |
| R | UNITED KINGDOM MODEL FOR END-STAGE LIVER DISEASE SCORE |
| R | CHILD PUGH SCORE |
| TREATMENT - LIVER |
|---|
| To carry other procedure details for Liver Metastasis and Liver Hepatocellular Carcinoma (HCC) for Liver cancer. One occurrence of this group is permitted per Core Treatment. | |
| M/R | Data Set Data Elements |
| R | ABLATIVE THERAPY TYPE |
| R | LIVER TRANSARTERIAL EMBOLISATION MATERIAL INJECTION TYPE |
| TRANSPLANTATION - LIVER |
|---|
| To carry liver transplantation details for Liver cancer. One occurrence of this group is permitted. | |
| M/R | Data Set Data Elements |
| R | LIVER TRANSPLANT WAITING LIST INDICATOR |
| TREATMENT: SURGERY - LIVER |
|---|
| To carry surgery details for Liver cancer. One occurrence of this group is permitted per Core Treatment Surgery. | |
| M/R | Data Set Data Elements |
| R | LIVER SURGERY PERFORMED TYPE |
Change to Data Set: Changed Description
Cancer Outcomes and Services Data Set Overview
Due to the rapidly changing situation with Covid-19, the implementation date of the Cancer Outcomes and Services Data Set (COSDS) has been deferred until 1 July 2020.
This will be reviewed and continued to be moved forward 3 months until there is a stable position nationally to resume the upgrade process.
For further information please contact: COSD@phe.gov.uk.
The previous version of the data set can be found at: COSDS Data Sets.
For a "Full Screen" view, click Cancer Outcomes and Services Data Set: Lung.
In the "Full Screen" view, to return to the "Data Set" view, click the browser "back" button.
The Mandatory or Required (M/R) column in the COSDS XML Schema indicates the recommendation for the inclusion of data.
- M = Mandatory: this data element is mandatory and the technical process (e.g. submission of the data set, production of output etc.) cannot be completed without this data element being present
- R = Required: NHS business processes cannot be delivered without this data element.
For guidance on downloading the XML Schema, see XML Schema TRUD Download.
For guidance on the XML Schema constraints, see the Cancer Outcomes and Services Data Set XML Schema Constraints.
| DIAGNOSTIC PROCEDURES CHOICE - LUNG |
|---|
| One of the following may be provided per Diagnostic Procedure | |
| CHOICE 1 - TRANSTHORACIC ECHOCARDIOGRAM | |
| To carry Transthoracic Echocardiogram details for the National Lung Cancer Audit (NLCA), to be captured once only for each care pathway for Lung cancer. One occurrence of this group is required per Core Diagnostic Procedures if selected as the choice. | |
| M/R | Data Set Data Elements |
| M | TRANSTHORACIC ECHOCARDIOGRAM TEST RESULT |
| CHOICE 2 - DIFFUSION CAPACITY | |
| To carry Diffusion Capacity details for the National Lung Cancer Audit (NLCA), to be captured once only for each care pathway for Lung cancer. One occurrence of this group is required per Core Diagnostic Procedures if selected as the choice. | |
| M/R | Data Set Data Elements |
| M | DIFFUSION CAPACITY TEST RESULT |
| CHOICE 3 - FEV1 | |
| To carry FEV1 details for the National Lung Cancer Audit (NLCA), to be captured once only for each care pathway for Lung cancer. One occurrence of this group is required per Core Diagnostic Procedures if selected as the choice. | |
| M/R | Data Set Data Elements |
| R | FORCED EXPIRATORY VOLUME IN 1 SECOND (PERCENTAGE) |
| R | FORCED EXPIRATORY VOLUME IN 1 SECOND (ABSOLUTE AMOUNT) |
| CHOICE 4 - CARDIOPULMONARY TEST | |
| To carry Cardiopulmonary Test details required for the National Lung Cancer Audit (NLCA) for Lung cancer. One occurrence of this group is required per Core Diagnostic Procedures if selected as the choice. | |
| M/R | Data Set Data Elements |
| R | CARDIOPULMONARY EXERCISE TEST TYPE |
| R | CARDIOPULMONARY EXERCISE TEST RESULT |
| CHOICE 5 - BRONCHOSCOPY | |
| To carry bronchoscopy details for Lung cancer. One occurrence of this group is required per Core Diagnostic Procedures if selected as the choice. | |
| M/R | Data Set Data Elements |
| M | BRONCHOSCOPY PERFORMED TYPE |
| MEDIASTINAL SAMPLING - LUNG |
|---|
| To carry Mediastinal Sampling details for the National Lung Cancer Audit (NLCA), to be captured once only for each care pathway for Lung cancer. One occurrence of this group is permitted. | |
| M/R | Data Set Data Elements |
| R | MEDIASTINAL SAMPLING INDICATOR |
| MOLECULAR AND BIOMARKERS - SOMATIC TESTING FOR TARGETED THERAPY AND PERSONALISED MEDICINE - LUNG |
|---|
| To carry Molecular details for Lung cancer. One occurrence of this group is permitted per Core - Molecular and Biomarkers - Somatic Testing for Targeted Therapy and Personalised Medicine. | |
| M/R | Data Set Data Elements |
| R | EPIDERMAL GROWTH FACTOR RECEPTOR MUTATIONAL STATUS |
| R | ALK GENE FUSION STATUS (LUNG CANCER) |
| R | ROS1 FUSION STATUS |
| R | PD-L1 EXPRESSION PERCENTAGE |
| TREATMENT - SURGERY: LUNG CANCER CONSULTANT OUTCOME PUBLICATION (LCCOP) - LUNG |
|---|
| To carry surgery details for the Lung Cancer Consultant Outcome Publication (LCCOP) for Lung cancer. One occurrence of this group is permitted per Core Treatment Surgery. | |
| M/R | Data Set Data Elements |
| R | REGIONAL ANAESTHETIC TECHNIQUE (CANCER) |
Change to Data Set: Changed Description
Cancer Outcomes and Services Data Set Overview
Due to the rapidly changing situation with Covid-19, the implementation date of the Cancer Outcomes and Services Data Set (COSDS) has been deferred until 1 July 2020.
This will be reviewed and continued to be moved forward 3 months until there is a stable position nationally to resume the upgrade process.
For further information please contact: COSD@phe.gov.uk.
The previous version of the data set can be found at: COSDS Data Sets.
For a "Full Screen" view, click Cancer Outcomes and Services Data Set: Pathology.
In the "Full Screen" view, to return to the "Data Set" view, click the browser "back" button.
The Mandatory, Required or Optional (M/R/O) column in the COSDS XML Schema indicates the recommendation for the inclusion of data.
- M = Mandatory: this data element is mandatory and the technical process (e.g. submission of the data set, production of output etc.) cannot be completed without this data element being present
- R = Required: NHS business processes cannot be delivered without this data element
- O = Optional: the inclusion of this data element is optional as required for local purposes.
For guidance on downloading the XML Schema, see XML Schema TRUD Download.
For guidance on the XML Schema constraints, see the Cancer Outcomes and Services Data Set XML Schema Constraints.
| SUBMISSION HEADER |
|---|
| To carry the submission header details. One occurrence of this group is required. | |
| M/R/O | Data Set Data Elements |
| M | COSDS SUBMISSION IDENTIFIER |
| M | ORGANISATION IDENTIFIER (CODE OF SUBMITTING ORGANISATION) |
| M | COSDS SUBMISSION RECORD COUNT |
| M | REPORTING PERIOD START DATE |
| M | REPORTING PERIOD END DATE |
| M | DATE AND TIME DATA SET CREATED |
| RECORD IDENTIFIER |
|---|
| To carry the record identifier details. One occurrence of this group is required. | |
| M/R/O | Data Set Data Elements |
| M | COSDS UNIQUE IDENTIFIER |
| LINKAGE IDENTIFIER CHOICE - CORE - PATHOLOGY |
|---|
| One of the following Core Linkage sections MUST be provided | |
| CHOICE 1 - NHS NUMBER | |
| To carry patient identity details for linkage. One occurrence of this group is required. | |
| M/R/O | Data Set Data Elements |
| M | NHS NUMBER |
| CHOICE 2 - LOCAL PATIENT IDENTIFIER | |
| To carry patient identity details for linkage. One occurrence of this group is required. | |
| M/R/O | Data Set Data Elements |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| LINKAGE IDENTIFIER - CORE - PATHOLOGY |
|---|
| To carry patient identity details for linkage. One occurrence of this group is required. | |
| M/R/O | Data Set Data Elements |
| M | NHS NUMBER STATUS INDICATOR CODE |
| M | PERSON BIRTH DATE |
| M | ORGANISATION IDENTIFIER (CODE OF PROVIDER) |
| DEMOGRAPHICS - CORE - PATHOLOGY |
|---|
| To carry patient demographic details. One occurrence of this group is permitted. | |
| M/R/O | Data Set Data Elements |
| R | PERSON FAMILY NAME |
| R | PERSON GIVEN NAME |
| R | PATIENT USUAL ADDRESS (AT DIAGNOSIS) - ADDRESS STRUCTURED or PATIENT USUAL ADDRESS (AT DIAGNOSIS) - ADDRESS UNSTRUCTURED |
| R | POSTCODE OF USUAL ADDRESS (AT DIAGNOSIS) |
| R | PERSON STATED GENDER CODE |
| PATHOLOGY DETAILS - CORE - PATHOLOGY |
|---|
| TOPOGRAPHY/MORPHOLOGY - CORE - PATHOLOGY |
|---|
| To carry Topography/Morphology SNOMED pathology details. One occurrence of this group is permitted. | |
| M/R/O | Data Set Data Elements |
| M | SNOMED VERSION (PATHOLOGY) |
| TOPOGRAPHY/MORPHOLOGY CHOICE - CORE - PATHOLOGY |
|---|
| One of the following MUST be provided | |
| CHOICE 1 - TOPOGRAPHY SNOMED | |
| To carry pathology Topography SNOMED details. One occurrence of this group is required. | |
| M/R/O | Data Set Data Elements |
| M | TOPOGRAPHY (SNOMED PATHOLOGY) Multiple occurrences of this item are permitted |
| CHOICE 2 - MORPHOLOGY SNOMED | |
| To carry pathology Morphology SNOMED details. One occurrence of this group is required. | |
| M/R/O | Data Set Data Elements |
| M | MORPHOLOGY (SNOMED PATHOLOGY) Multiple occurrences of this item are permitted |
| PATHOLOGY DETAILS - CORE - PATHOLOGY |
|---|
| BREAST - PATHOLOGY |
|---|
| CENTRAL NERVOUS SYSTEM (CNS) - PATHOLOGY |
|---|
| To carry pathology details for Central Nervous System (CNS) cancer. One occurrence of this data group is permitted. | |
| M/R/O | Data Set Data Elements |
| R | MOLECULAR DIAGNOSTIC CODE Multiple occurrences of this item are permitted |
| R | HORMONE EXPRESSION TYPE Multiple occurrences of this item are permitted |
| COLORECTAL - PATHOLOGY |
|---|
| To carry pathology details for Colorectal cancer. One occurrence of this data group is permitted. | |
| M/R/O | Data Set Data Elements |
| R | MARGIN INVOLVED INDICATION CODE (COLORECTAL PROXIMAL OR DISTAL RESECTION MARGIN) |
| R | DISTANCE TO CLOSEST NON PERITONEALISED RESECTION MARGIN |
| R | PLANE OF SURGICAL EXCISION INDICATOR |
| R | DISTANCE FROM DENTATE LINE |
| R | DISTANCE BEYOND MUSCULARIS PROPRIA |
| R | PREOPERATIVE THERAPY RESPONSE TYPE |
| R | MARGIN INVOLVED INDICATION CODE (CIRCUMFERENTIAL MARGIN) |
| CHILDREN, TEENAGERS AND YOUNG ADULTS (CTYA): RENAL PATHOLOGY (PAEDIATRIC KIDNEY) - PATHOLOGY |
|---|
| To carry renal (paediatric kidney) pathology details for Children, Teenagers, and Young Adults (CTYA) cancer. One occurrence of this data group is permitted. | |
| M/R/O | Data Set Data Elements |
| R | TUMOUR RUPTURE INDICATOR (PATHOLOGICAL) |
| R | ANAPLASTIC NEPHROBLASTOMA TYPE |
| R | TUMOUR INVASION INDICATOR (PERIRENAL FAT) |
| R | TUMOUR INVASION INDICATOR (RENAL SINUS) |
| R | RENAL VEIN TUMOUR INDICATOR (PAEDIATRIC KIDNEY) |
| R | VIABLE TUMOUR EVIDENCE AT RESECTION MARGIN |
| R | INTERNATIONAL SOCIETY OF PAEDIATRIC ONCOLOGY TUMOUR LOCAL STAGE |
| GYNAECOLOGICAL - PATHOLOGY |
|---|
| To carry pathology details for Gynaecological cancer. One occurrence of this data group is permitted. | |
| M/R/O | Data Set Data Elements |
| R | MICROSCOPIC INVOLVEMENT INDICATION CODE (FALLOPIAN TUBE) |
| R | MICROSCOPIC INVOLVEMENT INDICATION CODE (OVARIAN) |
| R | MICROSCOPIC INVOLVEMENT INDICATION CODE (UTERINE SEROSA) |
| R | OMENTUM INVOLVEMENT INDICATION CODE |
| GYNAECOLOGICAL: FALLOPIAN TUBE, OVARIAN EPITHELIAL AND PRIMARY PERITONEAL - PATHOLOGY |
|---|
| To carry pathology details for Gynaecological cancer for Fallopian Tube, Ovarian Epithelial and Primary Peritoneal. One occurrence of this group is permitted. | |
| M/R/O | Data Set Data Elements |
| R | GYNAECOLOGICIAL CAPSULE STATUS |
| R | OVARY SURFACE INVOLVEMENT INDICATOR |
| R | PERITONEAL CYTOLOGY RESULT CODE |
| R | PERITONEAL INVOLVEMENT INDICATION CODE |
| GYNAECOLOGICAL: ENDOMETRIAL - PATHOLOGY |
|---|
| To carry pathology details for Gynaecological cancer for Endometrial. One occurrence of this group is permitted. | |
| M/R/O | Data Set Data Elements |
| R | MICROSCOPIC INVOLVEMENT INDICATOR (CERVICAL STROMA) |
| R | MYOMETRIAL INVASION IDENTIFICATION CODE |
| R | MICROSCOPIC INVOLVEMENT INDICATOR (PARAMETRIUM) |
| R | PERITONEAL WASHINGS IDENTIFIED |
| R | PERITONEAL INVOLVEMENT INDICATOR (ENDOMETRIAL CANCER) |
| R | GYNAECOLOGICIAL CANCER SITE OF PERITONEAL INVOLVEMENT |
| GYNAECOLOGICAL: CERVICAL - PATHOLOGY |
|---|
| To carry pathology details for Gynaecological cancer for Cervical. One occurrence of this group is permitted. | |
| M/R/O | Data Set Data Elements |
| R | CERVICAL GLANDULAR INTRAEPITHELIAL NEOPLASIA PRESENCE AND GRADE |
| R | CERVICAL INTRAEPITHELIAL NEOPLASIA PRESENCE AND GRADE |
| R | SMILE INDICATION CODE |
| R | RESECTION MARGIN INVOLVEMENT INDICATOR |
| R | PARACERVICAL OR PARAMETRIAL INVOLVEMENT INDICATOR |
| R | UNINVOLVED CERVICAL STROMA THICKNESS |
| R | MICROSCOPIC INVOLVEMENT INDICATOR (VAGINAL) |
| R | INVASIVE THICKNESS |
| GYNAECOLOGICAL: NODES - PATHOLOGY |
|---|
| To carry pathology details for Gynaecological cancer for Nodes. One occurrence of this group is permitted. | |
| M/R/O | Data Set Data Elements |
| R | NUMBER OF NODES EXAMINED (PARA-AORTIC) |
| R | NUMBER OF NODES POSITIVE (PARA-AORTIC) |
| R | NUMBER OF NODES EXAMINED (PELVIC) |
| R | NUMBER OF NODES POSITIVE (PELVIC) |
| R | NUMBER OF NODES EXAMINED (INGUINO-FEMORAL) |
| R | NUMBER OF NODES POSITIVE (INGUINO-FEMORAL) |
| R | EXTRANODAL SPREAD INDICATOR |
| HEAD AND NECK: VARIOUS - PATHOLOGY |
|---|
| To carry pathology details for various Head and Neck cancers. One occurrence of this group is permitted. | |
| M/R/O | Data Set Data Elements |
| R | MAXIMUM DEPTH OF INVASION |
| R | BONE INVASION INDICATION CODE |
| R | CARTILAGE INVASION INDICATION CODE |
| R | ANATOMICAL SIDE (NECK DISSECTION) |
| HEAD AND NECK: SALIVARY - PATHOLOGY |
|---|
| To carry salivary pathology details for Head and Neck cancer. One occurrence of this group is permitted. | |
| M/R/O | Data Set Data Elements |
| M | MACROSCOPIC EXTRAGLANDULAR EXTENSION INDICATION CODE |
| HEAD AND NECK: GENERAL AND SALIVARY - PATHOLOGY |
|---|
| To carry general and salivary pathology details for Head and Neck cancer. One occurrence of this group is permitted. | |
| M/R/O | Data Set Data Elements |
| R | ANATOMICAL SIDE (POSITIVE NODES) |
| R | LARGEST METASTASIS (LEFT NECK) |
| R | LARGEST METASTASIS (RIGHT NECK) |
| R | EXTRACAPSULAR SPREAD INDICATION CODE |
| HEAD AND NECK: HUMAN PAPILLOMAVIRUS (HPV) - PATHOLOGY |
|---|
| To carry human papilloma virus pathology details for Head and Neck cancer. One occurrence of this group is permitted. | |
| M/R/O | Data Set Data Elements |
| R | P16 IMMUNOHISTOCHEMISTRY TEST RESULT |
| R | HUMAN PAPILLOMAVIRUS IN SITU HYBRIDISATION TEST RESULT |
| LUNG - PATHOLOGY |
|---|
| To carry pathology details for Lung cancer. One occurrence of this data group is permitted. | |
| M/R/O | Data Set Data Elements |
| R | EXTENT OF ATELECTASIS |
| R | EXTENT OF PLEURAL INVASION |
| R | TUMOUR INVASION INDICATOR (PERICARDIUM) |
| R | TUMOUR INVASION INDICATOR (DIAPHRAGM) |
| R | TUMOUR INVASION INDICATOR (GREAT VESSELS) |
| R | TUMOUR INVASION INDICATOR (HEART) |
| R | MALIGNANT PLEURAL EFFUSION INDICATOR |
| R | TUMOUR INVASION INDICATOR (MEDIASTINUM) |
| R | SATELLITE TUMOUR NODULES LOCATION |
| SARCOMA: BONE AND SOFT TISSUE - PATHOLOGY |
|---|
| To carry pathology details for Sarcoma for Bone and Soft Tissue. One occurrence of this data group is permitted. | |
| M/R/O | Data Set Data Elements |
| R | GENETIC CONFIRMATION INDICATOR |
| SARCOMA: BONE - PATHOLOGY |
|---|
| To carry pathology details for Sarcoma for Bone. One occurrence of this group is permitted. | |
| M/R/O | Data Set Data Elements |
| R | SARCOMA TUMOUR BREACH IDENTIFIER |
| R | TUMOUR NECROSIS PERCENTAGE |
| SARCOMA: SOFT TISSUE - PATHOLOGY |
|---|
| To carry pathology details for Sarcoma for Soft Tissue. One occurrence of this group is permitted. | |
| M/R/O | Data Set Data Elements |
| R | SARCOMA TUMOUR DEPTH |
| R | MITOTIC RATE (SARCOMA) |
| SKIN: BASAL CELL CARCINOMAS (BCC) - PATHOLOGY |
|---|
| To carry pathology details for Basal Cell Carcinoma (BCC) for skin cancer. One occurrence of this group is required. | |
| M/R/O | Data Set Data Elements |
| R | SKIN CANCER LESION SPECIMEN IDENTIFIER |
| R | PERINEURAL INVASION INDICATOR (SKIN) |
| R | LESION DIAMETER GREATER THAN 20MM INDICATION CODE |
| SKIN: SQUAMOUS CELL CARCINOMA (SCC) - PATHOLOGY |
|---|
| To carry pathology details for Squamous Cell Carcinoma (SCC) for skin cancer. One occurrence of this group is required. | |
| M/R/O | Data Set Data Elements |
| R | SKIN CANCER LESION SPECIMEN IDENTIFIER |
| R | PERINEURAL INVASION INDICATOR (SKIN) |
| R | LESION DIAMETER GREATER THAN 20MM INDICATION CODE |
| R | CLARKS LEVEL IV INDICATION CODE |
| R | LESION VERTICAL THICKNESS GREATER THAN 2MM INDICATION CODE |
| SKIN: MALIGNANT MELANOMA (MM) - PATHOLOGY |
|---|
| To carry pathology details for Malignant Melanoma (MM) for skin cancer. One occurrence of this group is permitted. | |
| M/R/O | Data Set Data Elements |
| R | SKIN CANCER LESION SPECIMEN IDENTIFIER |
| R | SKIN ULCERATION INDICATION CODE |
| R | MITOTIC RATE (SKIN) |
| R | MICROSATELLITE OR IN-TRANSIT METASTASIS INDICATION CODE |
| R | TUMOUR REGRESSION INDICATION CODE (SKIN) |
| R | BRESLOW THICKNESS |
| R | TUMOUR INFILTRATING LYMPHOCYTE TYPE |
| R | NUMBER OF SENTINEL NODES SAMPLED |
| R | NUMBER OF SENTINEL NODES POSITIVE |
| R | NUMBER OF NODES SAMPLED (POST SENTINEL NODE COMPLETION LYMPHADENECTOMY) |
| R | NUMBER OF NODES POSITIVE (POST SENTINEL NODE COMPLETION LYMPHADENECTOMY) |
| UPPER GASTROINTESTINAL (GI): VARIOUS - PATHOLOGY |
|---|
| To carry pathology details for various Upper Gastrointestinal (GI) cancers. One occurrence of this data group is permitted. | |
| M/R/O | Data Set Data Elements |
| R | NUMBER OF COLORECTAL METASTASES IN LIVER |
| R | MARGIN INVOLVED INDICATION CODE (PROXIMAL OR DISTAL RESECTION MARGIN) |
| R | MARGIN INVOLVED INDICATION CODE (CIRCUMFERENTIAL MARGIN) |
| UROLOGICAL: BLADDER - PATHOLOGY |
|---|
| To carry pathology details for Urological cancer for the bladder. One occurrence of this group is permitted. | |
| M/R/O | Data Set Data Elements |
| R | DETRUSOR MUSCLE PRESENCE INDICATION CODE |
| R | TUMOUR GRADE (UROLOGY) |
| UROLOGICAL: KIDNEY - PATHOLOGY |
|---|
| To carry pathology details for Urological cancer for the kidney. One occurrence of this group is permitted. | |
| M/R/O | Data Set Data Elements |
| R | TUMOUR NECROSIS INDICATION CODE |
| R | TUMOUR INVASION INDICATOR (PERINEPHRIC FAT) |
| R | TUMOUR INVASION INDICATION CODE (DIRECT ADRENAL) |
| R | RENAL VEIN TUMOUR THROMBUS INDICATION CODE (UROLOGICAL) |
| R | TUMOUR INVASION INDICATOR (GEROTAS FASCIA) |
| UROLOGICAL: PENIS - PATHOLOGY |
|---|
| To carry pathology details for Urological cancer for the penis. One occurrence of this group is permitted. | |
| M/R/O | Data Set Data Elements |
| R | TUMOUR INVASION INDICATOR (CORPUS SPONGIOSUM) |
| R | TUMOUR INVASION INDICATOR (CORPUS CAVERNOSUM) |
| R | TUMOUR INVASION INDICATOR (URETHRA OR PROSTATE) |
| UROLOGICAL: PROSTATE - PATHOLOGY |
|---|
| To carry pathology details for Urological cancer for prostate. One occurrence of this group is permitted. | |
| M/R/O | Data Set Data Elements |
| R | GLEASON GRADE (PRIMARY) |
| R | GLEASON GRADE (SECONDARY) |
| R | GLEASON GRADE (TERTIARY) |
| R | PERINEURAL INVASION INDICATOR (UROLOGICAL) |
| R | TURP TUMOUR PERCENTAGE |
| UROLOGICAL: TESTICULAR - PATHOLOGY |
|---|
| To carry pathology details for Urological cancer for testicular. One occurrence of this group is permitted. | |
| M/R/O | Data Set Data Elements |
| M | TUMOUR INVASION INDICATOR (RETE TESTIS) |
Change to Data Set: Changed Description
Cancer Outcomes and Services Data Set Overview
Due to the rapidly changing situation with Covid-19, the implementation date of the Cancer Outcomes and Services Data Set (COSDS) has been deferred until 1 July 2020.
This will be reviewed and continued to be moved forward 3 months until there is a stable position nationally to resume the upgrade process.
For further information please contact: COSD@phe.gov.uk.
The previous version of the data set can be found at: COSDS Data Sets.
For a "Full Screen" view, click Cancer Outcomes and Services Data Set: Sarcoma.
In the "Full Screen" view, to return to the "Data Set" view, click the browser "back" button.
The Mandatory or Required (M/R) column in the COSDS XML Schema indicates the recommendation for the inclusion of data.
- M = Mandatory: this data element is mandatory and the technical process (e.g. submission of the data set, production of output etc.) cannot be completed without this data element being present
- R = Required: NHS business processes cannot be delivered without this data element.
For guidance on downloading the XML Schema, see XML Schema TRUD Download.
For guidance on the XML Schema constraints, see the Cancer Outcomes and Services Data Set XML Schema Constraints.
| DIAGNOSIS - SARCOMA |
|---|
| To carry diagnostic details for Bone and Soft Tissue for Sarcoma cancer. One occurrence of this group is permitted per Core Diagnosis. | |
| M/R | Data Set Data Elements |
| R | SARCOMA TUMOUR SITE (BONE) |
| R | SARCOMA TUMOUR SUBSITE (BONE) |
| R | SARCOMA TUMOUR SITE (SOFT TISSUE) |
| R | SARCOMA TUMOUR SUBSITE (SOFT TISSUE) |
| R | MULTIFOCAL OR SYNCHRONOUS TUMOUR INDICATOR |
| DIAGNOSIS CHOICE - SARCOMA |
|---|
| One of the following can be provided per Core Diagnosis | |
| CHOICE 1 - RHABDOMYOSARCOMA AND OTHER SOFT TISSUE SARCOMAS | |
| To carry diagnostic Rhabdomyosarcoma and other Soft Tissue Sarcoma details for Sarcoma cancer. One occurrence of this group is required per Core Diagnosis if selected as the choice. | |
| M/R | Data Set Data Elements |
| R | INTERGROUP RHABDOMYOSARCOMA STUDY POST SURGICAL GROUP |
| R | INTERGROUP RHABDOMYOSARCOMA STUDY POST SURGICAL GROUP DATE |
| R | RHABDOMYOSARCOMA SITE PROGNOSIS CODE |
| CHOICE 2 - EWINGS | |
| To carry diagnostic Ewings details for Sarcoma cancer. One occurrence of this group is required per Core Diagnosis if selected as the choice. | |
| M/R | Data Set Data Elements |
| M | TUMOUR VOLUME AT DIAGNOSIS CODE |
| LABORATORY RESULTS CHOICE - SARCOMA |
|---|
| One of the following can be provided per Core Laboratory Results | |
| CHOICE 1 - RHABDOMYOSARCOMA AND OTHER SOFT TISSUE SARCOMAS | |
| To carry Rhabdomyosarcoma and other Soft Tissue Sarcoma laboratory result details for Sarcoma cancer. One occurrence of this group is required per Core Laboratory Results if selected as the choice. | |
| M/R | Data Set Data Elements |
| M | CYTOGENETIC PRESENCE TYPE (RHABDOMYOSARCOMA) |
| CHOICE 2 - EWINGS | |
| To carry Ewings laboratory result details for Sarcoma cancer. One occurrence of this group is required per Core Laboratory Results if selected as the choice. | |
| M/R | Data Set Data Elements |
| M | CYTOGENETIC ANALYSIS CODE |
Change to Data Set: Changed Description
Cancer Outcomes and Services Data Set Overview
Due to the rapidly changing situation with Covid-19, the implementation date of the Cancer Outcomes and Services Data Set (COSDS) has been deferred until 1 July 2020.
This will be reviewed and continued to be moved forward 3 months until there is a stable position nationally to resume the upgrade process.
For further information please contact: COSD@phe.gov.uk.
The previous version of the data set can be found at: COSDS Data Sets.
For a "Full Screen" view, click Cancer Outcomes and Services Data Set: Skin.
In the "Full Screen" view, to return to the "Data Set" view, click the browser "back" button.
The Mandatory or Required (M/R) column in the COSDS XML Schema indicates the recommendation for the inclusion of data.
- M = Mandatory: this data element is mandatory and the technical process (e.g. submission of the data set, production of output etc.) cannot be completed without this data element being present
- R = Required: NHS business processes cannot be delivered without this data element.
For guidance on downloading the XML Schema, see XML Schema TRUD Download.
For guidance on the XML Schema constraints, see the Cancer Outcomes and Services Data Set XML Schema Constraints.
| TREATMENT - SURGERY: BASAL CELL CARCINOMAS (BCC), SQUAMOUS CELL CARCINOMA (SCC), MALIGNANT MELANOMA (MM) - SKIN |
|---|
| To carry surgery details for Basal Cell Carcinoma (BCC), Squamous Cell Carcinoma (SCC) and Malignant Melanoma (MM) for Skin cancer. One occurrence of this group is permitted per Core Treatment Surgery. | |
| M/R | Data Set Data Elements |
| R | CARE PROFESSIONAL OPERATING SURGEON TYPE (CANCER) |
| R | MEMBER OF SPECIALIST MULTIDISCIPLINARY TEAM INDICATOR |
Change to Data Set: Changed Description
Cancer Outcomes and Services Data Set Overview
Due to the rapidly changing situation with Covid-19, the implementation date of the Cancer Outcomes and Services Data Set (COSDS) has been deferred until 1 July 2020.
This will be reviewed and continued to be moved forward 3 months until there is a stable position nationally to resume the upgrade process.
For further information please contact: COSD@phe.gov.uk.
The previous version of the data set can be found at: COSDS Data Sets.
For a "Full Screen" view, click Cancer Outcomes and Services Data Set: Upper Gastrointestinal.
In the "Full Screen" view, to return to the "Data Set" view, click the browser "back" button.
The Mandatory or Required (M/R) column in the COSDS XML Schema indicates the recommendation for the inclusion of data.
- M = Mandatory: this data element is mandatory and the technical process (e.g. submission of the data set, production of output etc.) cannot be completed without this data element being present
- R = Required: NHS business processes cannot be delivered without this data element.
For guidance on downloading the XML Schema, see XML Schema TRUD Download.
For guidance on the XML Schema constraints, see the Cancer Outcomes and Services Data Set XML Schema Constraints.
| TREATMENT - SURGERY: GENERAL - UPPER GASTROINTESTINAL (GI) |
|---|
| To carry surgery details for Upper Gastrointestinal (GI) cancer. One occurrence of this group is permitted per Core Treatment Surgery. | |
| M/R | Data Set Data Elements |
| M | PALLIATIVE TREATMENT REASON (UPPER GASTROINTESTINAL) |
| TREATMENT - SURGERY: OESO-GASTRIC - UPPER GASTROINTESTINAL (GI) |
|---|
| To carry surgery details for Oeso-Gastric for Upper Gastrointestinal (GI) cancer. One occurrence of this group is permitted per Core Treatment Surgery. | |
| M/R | Data Set Data Elements |
| M | POST OPERATIVE TUMOUR SITE (UPPER GASTROINTESTINAL) |
| TREATMENT - SURGERY: ESODATA - UPPER GASTROINTESTINAL (GI) |
|---|
| To carry surgery details for the Esophageal Database (ESODATA) for Upper Gastrointestinal (GI) cancer. One occurrence of this group is permitted per Core Treatment Surgery. | |
| M/R | Data Set Data Elements |
| R | INTERNATIONAL ESOPHAGEAL DATABASE SURGICAL COMPLICATIONS Multiple occurrences of this item are permitted |
| R | OESOPHAGOENTERIC LEAK SEVERITY TYPE |
| R | OESOPHAGECTOMY OESOPHAGEAL CONDUIT NECROSIS FAILURE TYPE |
| R | RECURRENT LARYNGEAL NERVE INJURY INVOLVEMENT TYPE |
| R | CHYLE LEAK SEVERITY TYPE |
| R | CALVIEN-DINDO CLASSIFICATION OF SURGICAL CLASSIFICATIONS |
| R | ADDITIONAL INTERNATIONAL ESOPHAGEAL DATABASE SURGICAL COMPLICATIONS Multiple occurrences of this item are permitted |
| TREATMENT - SURGERY: OUTCOME MEASURES - UPPER GASTROINTESTINAL (GI) |
|---|
| To carry surgery outcome measures for the Esophageal Database (ESODATA) for Upper Gastrointestinal (GI) cancer. One occurrence of this group is permitted per Core Treatment Surgery. | |
| M/R | Data Set Data Elements |
| R | ESCALATION IN LEVEL OF PATIENT CARE FOLLOWING OESOPHAGECTOMY INDICATOR |
| R | BLOOD PRODUCTS REQUIRED FOLLOWING OESOPHAGECTOMY INDICATION CODE |
| R | UNITS OF BLOOD TRANSFUSED FOLLOWING OESOPHAGECTOMY |
| TREATMENT - SURGERY: OESOPHAGECTOMY - UPPER GASTROINTESTINAL (GI) |
|---|
| To carry surgery details for the Oesophagectomy for Upper Gastrointestinal (GI) cancer. One occurrence of this group is permitted per Core Treatment Surgery. | |
| M/R | Data Set Data Elements |
| R | OESOPHAGECTOMY SURGICAL APPROACH TYPE |
| R | OPEN OESOPHAGECTOMY SURGICAL APPROACH TYPE |
| R | MINIMALLY INVASIVE OESOPHAGECTOMY SURGICAL APPROACH TYPE |
| R | OESOPHAGECTOMY ANASTOMOSIS TYPE |
| R | OESOPHAGECTOMY OESOPHAGEAL CONDUIT TYPE |
| R | OESOPHAGECTOMY NECK DISSECTION INDICATOR |
| TREATMENT - SURGERY: LIVER CHOLANGIOCARCINOMA AND PANCREATIC - UPPER GASTROINTESTINAL (GI) |
|---|
| To carry surgery details for Liver Cholangiocarcinoma and Pancreatic for Upper Gastrointestinal (GI) cancer. One occurrence of this group is permitted per Core Treatment Surgery. | |
| M/R | Data Set Data Elements |
| M | SURGICAL PALLIATION TYPE |
| TREATMENT - SURGERY: ENDOSCOPIC OR RADIOLOGICAL PROCEDURES (PANCREATIC AND OESO-GASTRIC) - UPPER GASTROINTESTINAL (GI) |
|---|
| To carry surgery details for endoscopic and radiological procedures for Pancreatic and Oeso-Gastric for Upper Gastrointestinal (GI) cancer. One occurrence of this group is permitted per Core Treatment Surgery. | |
| M/R | Data Set Data Elements |
| M | ENDOSCOPIC PROCEDURE TYPE Multiple occurrences of this item are permitted |
| TREATMENT - SURGERY: ENDOSCOPIC OR RADIOLOGICAL PROCEDURES (MAIN) - UPPER GASTROINTESTINAL (GI) |
|---|
| To carry surgery details for endoscopic and radiological procedures for Upper Gastrointestinal (GI) cancer. One occurrence of this group is permitted per Core Treatment Surgery. | |
| M/R | Data Set Data Elements |
| M | ENDOSCOPIC OR RADIOLOGICAL COMPLICATION TYPE Multiple occurrences of this item are permitted |
Change to Data Set: Changed Description
Cancer Outcomes and Services Data Set Overview
Due to the rapidly changing situation with Covid-19, the implementation date of the Cancer Outcomes and Services Data Set (COSDS) has been deferred until 1 July 2020.
This will be reviewed and continued to be moved forward 3 months until there is a stable position nationally to resume the upgrade process.
For further information please contact: COSD@phe.gov.uk.
The previous version of the data set can be found at: COSDS Data Sets.
For a "Full Screen" view, click Cancer Outcomes and Services Data Set: Urological.
In the "Full Screen" view, to return to the "Data Set" view, click the browser "back" button.
The Mandatory or Required (M/R) column in the COSDS XML Schema indicates the recommendation for the inclusion of data.
- M = Mandatory: this data element is mandatory and the technical process (e.g. submission of the data set, production of output etc.) cannot be completed without this data element being present
- R = Required: NHS business processes cannot be delivered without this data element.
For guidance on downloading the XML Schema, see XML Schema TRUD Download.
For guidance on the XML Schema constraints, see the Cancer Outcomes and Services Data Set XML Schema Constraints.
| DIAGNOSTIC PROCEDURES: PROSTATE - UROLOGICAL |
|---|
| To carry cancer diagnostic procedure details for Urological cancer for the prostate. One occurrence of this group is permitted per Core Diagnostic Procedures. | |
| M/R | Data Set Data Elements |
| M | PRETREATMENT PROSTATE BIOPSY TECHNIQUE TYPE |
| M | BIOPSY ANAESTHETIC TYPE |
| DIAGNOSIS: PROSTATE - UROLOGICAL |
|---|
| To carry the diagnosis details for Urological cancer for the prostate. One occurrence of this group is permitted per Core Diagnosis. | |
| M/R | Data Set Data Elements |
| R | MULTIPARAMETRIC MRI SCAN INDICATOR |
| R | MRI ULTRASOUND FUSION GUIDED BIOPSY INDICATOR |
| R | PROSTATE SPECIFIC ANTIGEN (DIAGNOSIS) |
| CANCER CARE PLAN - UROLOGICAL |
|---|
| To carry cancer care plan details for Urological cancer. One occurrence of this group is permitted per Core Cancer Care Plan. | |
| M/R | Data Set Data Elements |
| R | ESTIMATED GLOMERULAR FILTRATION RATE |
| R | HYDRONEPHROSIS CODE |
| R | S CATEGORY CODE |
| LABORATORY RESULTS - UROLOGICAL |
|---|
| To carry Laboratory Result details for Urological cancer. One occurrence of this group is permitted per Core Laboratory Results. | |
| M/R | Data Set Data Elements |
| R | S CATEGORY (ALPHA FETOPROTEIN) |
| R | S CATEGORY (HUMAN CHORIONIC GONADOTROPIN) |
| R | S CATEGORY (LACTATE DEHYDROGENASE) |
| R | LACTATE DEHYDROGENASE LEVEL (NORMAL UPPER LIMIT) |
| STAGING: TESTICULAR - UROLOGICAL |
|---|
| To carry staging details for Urological cancer for testicular. One occurrence of this group is permitted per Core Site Specific Staging. | |
| M/R | Data Set Data Elements |
| R | STAGE GROUPING (TESTICULAR CANCER) |
| R | EXTENT OF METASTATIC SPREAD Multiple occurrences of this item are permitted |
| R | LUNG METASTASES SUB-STAGE GROUPING |
| TREATMENT: BLADDER CHOICE - UROLOGICAL |
|---|
| One of the following can be provided per Urological Treatment | |
| CHOICE 1 - INTRAVESICAL CHEMOTHERAPY | |
| To carry treatment details for Urological cancer for the bladder. One occurrence of this group is required per Core Treatment if selected as the choice. | |
| M/R | Data Set Data Elements |
| M | INTRAVESICAL CHEMOTHERAPY RECEIVED INDICATOR |
| CHOICE 2 - INTRAVESICAL IMMUNOTHERAPY | |
| To carry treatment details for Urological cancer for the bladder. One occurrence of this group is required per Core Treatment if selected as the choice. | |
| M/R | Data Set Data Elements |
| M | INTRAVESICAL IMMUNOTHERAPY RECEIVED INDICATOR |
| TREATMENT: PROSTATE - UROLOGICAL |
|---|
| To carry cancer treatment details for Urological cancer for the prostate. One occurrence of this group is required per Core Treatment. | |
| M/R | Data Set Data Elements |
| R | PROSTATE NERVE SPARING SURGERY TYPE |
| R | RADICAL PROSTATECTOMY MARGIN STATUS |
Change to Data Set: Changed Description
Community Services Data Set Overview
Due to the rapidly changing situation with Covid-19 for both providers and NHS Digital, the transition from Community Services Data Set v1.0 to v1.5 has been postponed until 1 July 2020.The situation will be reviewed regularly and any decision regarding a further extension will be made at least two weeks prior to the new go-live date.
For further information please contact: enquiries@nhsdigital.nhs.uk.
Version 1.0 of the data set can be found at: Community Services Data Set.
For a "Full Screen" view, click Community Services Data Set.
In the "Full Screen" view, to return to the "Data Set" view, click the browser "back" button.
The Mandatory, Required or Optional (M/R/O) column indicates the recommendation for the inclusion of data.
- M = Mandatory: this data element is mandatory and the technical process (e.g. submission of the data set, production of output etc) cannot be completed without this data element being present
- R = Required: NHS business processes cannot be delivered without this data element
- O = Optional: the inclusion of this data element is optional as required for local purposes.
For guidance on the Data Set constraints, see the Community Services Data Set Constraints.
For guidance on downloading the XML Schema, see XML Schema TRUD Download.
| SUBMISSION IDENTIFIER |
|---|
| PATIENT DEMOGRAPHICS |
|---|
| GP Practice Registration: To carry details of the GP Practice Registration of the patient. One occurrence of this group is required for each change of GP Practice Registration. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| M | GENERAL MEDICAL PRACTICE CODE (PATIENT REGISTRATION) |
| R | START DATE (GMP PATIENT REGISTRATION) |
| R | END DATE (GMP PATIENT REGISTRATION) |
| R | ORGANISATION IDENTIFIER (GP PRACTICE RESPONSIBILITY) |
| Accommodation Type: To carry details of the type of accommodation for the patient. One occurrence of this group is permitted for each accommodation status. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| M | ACCOMMODATION STATUS CODE |
| R | ACCOMMODATION STATUS RECORDED DATE |
| Care Plan Type: To carry details of Care Plans created for a patient by the organisation. One occurrence of this group is permitted for each Care Plan created for the patient. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | CARE PLAN IDENTIFIER |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| M | CARE PLAN TYPE (COMMUNITY CARE) |
| M | CARE PLAN CREATION DATE |
| R | CARE PLAN CREATION TIME |
| R | CARE PLAN LAST UPDATED DATE |
| R | CARE PLAN LAST UPDATED TIME |
| R | CARE PLAN IMPLEMENTATION DATE |
| Care Plan Agreement: To carry details of any agreements to a Care Plan by a patient, team or organisation. One occurrence of this group is permitted for each agreement of a Care Plan. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | CARE PLAN IDENTIFIER |
| M | CARE PLAN AGREED BY |
| R | CARE PLAN AGREED DATE |
| R | CARE PLAN AGREED TIME |
| Social and Personal Circumstances: To carry details of social and personal circumstances of a patient. One occurrence of this group is permitted for each social and personal circumstance recorded. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| M | SOCIAL AND PERSONAL CIRCUMSTANCE (SNOMED CT) |
| M | SOCIAL AND PERSONAL CIRCUMSTANCE RECORDED DATE |
| Employment Status: To carry details of the employment status of the patient. One occurrence of this group is permitted for each employment status. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| M | EMPLOYMENT STATUS |
| R | EMPLOYMENT STATUS RECORDED DATE |
| R | WEEKLY HOURS WORKED |
| REFERRALS |
|---|
| Service or Team Referral: To carry details of the Service or Team referral that the patient is subject to. One occurrence of this group is permitted for each referral. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | SERVICE REQUEST IDENTIFIER |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| M | ORGANISATION IDENTIFIER (CODE OF COMMISSIONER) |
| M | REFERRAL REQUEST RECEIVED DATE |
| R | REFERRAL REQUEST RECEIVED TIME |
| O | NHS SERVICE AGREEMENT LINE NUMBER |
| R | SOURCE OF REFERRAL FOR COMMUNITY |
| R | ORGANISATION IDENTIFIER (REFERRING) |
| R | REFERRING CARE PROFESSIONAL STAFF GROUP (MENTAL HEALTH AND COMMUNITY CARE) |
| R | PRIORITY TYPE CODE |
| R | PRIMARY REASON FOR REFERRAL (COMMUNITY CARE) |
| R | SERVICE DISCHARGE DATE |
| R | DISCHARGE LETTER ISSUED DATE (MENTAL HEALTH AND COMMUNITY CARE) |
| Service or Team Type Referred To: To carry details of the Service or Team that the patient has been referred to. One occurrence of this group is permitted for each service or team that a patient has been referred to. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | SERVICE REQUEST IDENTIFIER |
| R | CARE PROFESSIONAL TEAM LOCAL IDENTIFIER |
| M | SERVICE OR TEAM TYPE REFERRED TO (COMMUNITY CARE) |
| R | REFERRAL CLOSURE DATE |
| R | REFERRAL REJECTION DATE |
| R | REFERRAL CLOSURE REASON |
| R | REFERRAL REJECTION REASON |
| Other Reason for Referral: To carry details of additional reasons why a patient has been referred to a specific service. One occurrence of this group is permitted for each additional referral reason. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | SERVICE REQUEST IDENTIFIER |
| M | OTHER REASON FOR REFERRAL (COMMUNITY CARE) |
| Referral To Treatment (RTT): To carry Referral to Treatment details for the patient referral. One occurrence of this group is permitted for each change in Referral To Treatment Period Status. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | SERVICE REQUEST IDENTIFIER |
| R | UNIQUE BOOKING REFERENCE NUMBER (CONVERTED) |
| R | PATIENT PATHWAY IDENTIFIER |
| R | ORGANISATION IDENTIFIER (PATIENT PATHWAY IDENTIFIER ISSUER) |
| R | WAITING TIME MEASUREMENT TYPE |
| R | REFERRAL TO TREATMENT PERIOD START DATE |
| R | REFERRAL TO TREATMENT PERIOD START TIME |
| R | REFERRAL TO TREATMENT PERIOD END DATE |
| R | REFERRAL TO TREATMENT PERIOD END TIME |
| R | REFERRAL TO TREATMENT PERIOD STATUS |
| Onward Referral: To carry details of any onward referral of the patient which has taken place. One occurrence of this group is permitted for each onward referral. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | SERVICE REQUEST IDENTIFIER |
| M | ONWARD REFERRAL DATE |
| R | ONWARD REFERRAL REASON |
| R | ORGANISATION IDENTIFIER (RECEIVING) |
| CARE CONTACT AND ACTIVITIES |
|---|
| Care Contact: To carry details of any contacts with a patient which have taken place as result of a referral. One occurrence of this group is permitted for each Care Contact. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | CARE CONTACT IDENTIFIER |
| M | SERVICE REQUEST IDENTIFIER |
| R | CARE PROFESSIONAL TEAM LOCAL IDENTIFIER |
| M | CARE CONTACT DATE |
| R | CARE CONTACT TIME |
| R | ORGANISATION IDENTIFIER (CODE OF COMMISSIONER) |
| R | ADMINISTRATIVE CATEGORY CODE |
| R | CLINICAL CONTACT DURATION OF CARE CONTACT |
| R | CONSULTATION TYPE |
| R | CARE CONTACT SUBJECT |
| R | CONSULTATION MEDIUM USED |
| R | ACTIVITY LOCATION TYPE CODE |
| R | ORGANISATION SITE IDENTIFIER (OF TREATMENT) |
| R | GROUP THERAPY INDICATOR |
| R | ATTENDED OR DID NOT ATTEND CODE |
| R | EARLIEST REASONABLE OFFER DATE |
| R | EARLIEST CLINICALLY APPROPRIATE DATE |
| R | CARE CONTACT CANCELLATION DATE |
| R | CARE CONTACT CANCELLATION REASON |
| R | REPLACEMENT APPOINTMENT DATE OFFERED |
| R | REPLACEMENT APPOINTMENT BOOKED DATE |
| Care Activity: To carry details of any activities which have taken place as part of a contact with a patient. One occurrence of this group is permitted for each Care Activity. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | CARE ACTIVITY IDENTIFIER |
| M | CARE CONTACT IDENTIFIER |
| M | COMMUNITY CARE ACTIVITY TYPE |
| R | CARE PROFESSIONAL LOCAL IDENTIFIER |
| R | CLINICAL CONTACT DURATION OF CARE ACTIVITY |
| R | PROCEDURE SCHEME IN USE |
| R | CODED PROCEDURE (CLINICAL TERMINOLOGY) |
| R | FINDING SCHEME IN USE |
| R | CODED FINDING (CODED CLINICAL ENTRY) |
| R | OBSERVATION SCHEME IN USE |
| R | CODED OBSERVATION (CLINICAL TERMINOLOGY) |
| R | OBSERVATION VALUE |
| R | UCUM UNIT OF MEASUREMENT |
| GROUP SESSIONS |
|---|
| Group Session: To carry details of any group sessions which have been provided to a group of people during the reporting period. One occurrence of this group is permitted for each Group Session activity. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | GROUP SESSION IDENTIFIER |
| M | GROUP SESSION DATE |
| M | ORGANISATION IDENTIFIER (CODE OF COMMISSIONER) |
| R | CLINICAL CONTACT DURATION OF GROUP SESSION |
| R | GROUP SESSION TYPE (COMMUNITY CARE) |
| R | NUMBER OF GROUP SESSION PARTICIPANTS |
| O | ACTIVITY LOCATION TYPE CODE |
| R | ORGANISATION SITE IDENTIFIER (OF TREATMENT) |
| R | CARE PROFESSIONAL LOCAL IDENTIFIER |
| O | NHS SERVICE AGREEMENT LINE NUMBER |
| SOCIAL CIRCUMSTANCES |
|---|
| Special Educational Need Identified: To carry details of the child's or young person's Special Educational Need. One occurrence of this group is permitted for each Special Educational Need identified. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| M | SPECIAL EDUCATIONAL NEED TYPE |
| Safeguarding Vulnerability Factor: To carry details when the child's or young person is subject to any safeguarding concerns. One occurrence of this group is permitted for each safeguarding concern. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| M | SAFEGUARDING VULNERABILITY FACTORS TYPE |
| Child Protection Plan: To carry details when the child or young person is subject to a child protection plan. One occurrence of this group is permitted for each child protection plan. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| M | CHILD PROTECTION PLAN REASON CODE |
| M | CHILD PROTECTION PLAN START DATE |
| R | CHILD PROTECTION PLAN END DATE |
| Assistive Technology to Support Disability Type: To carry details when assistive technology is used to help support a disabled child or young person. One occurrence of this group is permitted for each assistive technology type. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| M | ASSISTIVE TECHNOLOGY FINDING (SNOMED CT) |
| R | PRESCRIPTION DATE (ASSISTIVE TECHNOLOGY) |
| IMMUNISATIONS |
|---|
| Coded Immunisation: To carry details of coded immunisation activity for a patient. One occurrence of this group is permitted for each coded immunisation activity. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| M | IMMUNISATION DATE |
| M | PROCEDURE SCHEME IN USE |
| M | IMMUNISATION PROCEDURE (CLINICAL TERMINOLOGY) |
| R | ORGANISATION IDENTIFIER (IMMUNISATION RESPONSIBLE ORGANISATION) |
| Immunisation: To carry details of immunisation activity for a child or young person. One occurrence of this group is permitted for each immunisation activity. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| M | IMMUNISATION DATE |
| M | CHILDHOOD IMMUNISATION TYPE (CHILDREN AND YOUNG PEOPLE'S HEALTH SERVICES) |
| R | ORGANISATION IDENTIFIER (IMMUNISATION RESPONSIBLE ORGANISATION) |
| DIAGNOSES, TESTS AND OBSERVATIONS |
|---|
| Medical History (Previous Diagnosis): To carry details of any previous diagnoses for a patient, which are stated by the patient or patient proxy or recorded in medical notes. These do not have to have been diagnosed by the organisation submitting the data. One occurrence of this group is permitted for each previous diagnosis. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| M | DIAGNOSIS SCHEME IN USE |
| M | PREVIOUS DIAGNOSIS (CODED CLINICAL ENTRY) |
| R | DIAGNOSIS DATE |
| Disability Type: To carry details of the type of disability affecting a patient, based on their perception or the perception of a patient proxy. One occurrence of this group is permitted for each disability identified. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| M | DISABILITY CODE |
| R | DISABILITY IMPACT PERCEPTION |
| Newborn Hearing Screening Audiology Referral: To carry details of how concerns following Newborn Hearing Screening are followed up. One occurrence of this group is permitted for each newborn hearing audiology test. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| R | NEWBORN HEARING SCREENING OUTCOME |
| R | SERVICE REQUEST DATE (NEWBORN HEARING AUDIOLOGY) |
| R | PROCEDURE DATE (NEWBORN HEARING AUDIOLOGY) |
| R | NEWBORN HEARING AUDIOLOGY OUTCOME |
| Infant Physical Examination (General Medical Practitioner Delivered): To carry details of the Infant Physical Examination carried out by the General Medical Practitioner. One occurrence of this group is permitted for each Infant Physical Examination. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| M | INFANT PHYSICAL EXAMINATION DATE |
| R | INFANT PHYSICAL EXAMINATION RESULT (HIPS) |
| R | INFANT PHYSICAL EXAMINATION RESULT (HEART) |
| R | INFANT PHYSICAL EXAMINATION RESULT (EYES) |
| R | INFANT PHYSICAL EXAMINATION RESULT (TESTES) |
| Provisional Diagnosis: To carry details of a provisional diagnosis for a patient made by the service that the patient was referred to. One occurrence of this group is permitted for each provisional diagnosis. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | SERVICE REQUEST IDENTIFIER |
| M | DIAGNOSIS SCHEME IN USE |
| M | PROVISIONAL DIAGNOSIS (CODED CLINICAL ENTRY) |
| R | PROVISIONAL DIAGNOSIS DATE |
| Primary Diagnosis: To carry details of the primary diagnosis for a patient made by the service that the patient was referred to. One occurrence of this group is permitted for the primary diagnosis. The primary diagnosis can change during a reporting period. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | SERVICE REQUEST IDENTIFIER |
| M | DIAGNOSIS SCHEME IN USE |
| M | PRIMARY DIAGNOSIS (CODED CLINICAL ENTRY) |
| R | DIAGNOSIS DATE |
| Secondary Diagnosis: To carry details of a secondary diagnosis for a patient made by the service that the patient was referred to. One occurrence of this group is permitted for each secondary diagnosis. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | SERVICE REQUEST IDENTIFIER |
| M | DIAGNOSIS SCHEME IN USE |
| M | SECONDARY DIAGNOSIS (CODED CLINICAL ENTRY) |
| R | DIAGNOSIS DATE |
| Coded Scored Assessment (Referral): To carry details of scored assessments that are issued and completed as part of a referral period where a specific service or team is responsible for the patient, but do not take place at a specific contact. One occurrence of this group is permitted for each coded scored assessment question or dimension captured outside of a contact. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | SERVICE REQUEST IDENTIFIER |
| M | CODED ASSESSMENT TOOL TYPE (SNOMED CT) |
| M | PERSON SCORE |
| R | ASSESSMENT TOOL COMPLETION DATE |
| Breastfeeding Status: To carry details of a child's breastfeeding status as recorded at a contact. One occurrence of this group is permitted containing the most recently recorded breastfeeding status. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | CARE ACTIVITY IDENTIFIER |
| M | BREASTFEEDING STATUS |
| Observation: To carry details of observations of a patient which take place at a contact. One occurrence of this group is permitted containing the most recently recorded observation(s). | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | CARE ACTIVITY IDENTIFIER |
| R | PERSON WEIGHT |
| R | PERSON HEIGHT IN METRES |
| R | PERSON LENGTH IN CENTIMETRES |
| Coded Scored Assessment (Contact): To carry details of scored assessments that are issued and completed as part of a specific contact. One occurrence of this group is permitted for each coded scored assessment question or dimension. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | CARE ACTIVITY IDENTIFIER |
| M | CODED ASSESSMENT TOOL TYPE (SNOMED CT) |
| M | PERSON SCORE |
| ANONYMOUS SELF-ASSESSMENT |
|---|
| Anonymous Self-Assessment: To carry details of anonymous assessments that are issued by the Community Health Service. One occurrence of this group is permitted when an anonymous self-assessment is received from a patient. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | ASSESSMENT TOOL COMPLETION DATE |
| M | CODED ASSESSMENT TOOL TYPE (SNOMED CT) |
| M | PERSON SCORE |
| R | ACTIVITY LOCATION TYPE CODE |
| R | ORGANISATION IDENTIFIER (CODE OF COMMISSIONER) |
| STAFF DETAILS |
|---|
| Staff Details: To carry details of the staff involved in the treatment of a patient. One occurrence of this group is permitted for each staff member. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | CARE PROFESSIONAL LOCAL IDENTIFIER |
| R | PROFESSIONAL REGISTRATION BODY CODE |
| R | PROFESSIONAL REGISTRATION ENTRY IDENTIFIER |
| R | CARE PROFESSIONAL STAFF GROUP (COMMUNITY CARE) |
| R | OCCUPATION CODE |
| R | CARE PROFESSIONAL (JOB ROLE CODE) |
Change to Data Set: Changed Description
The Improving Access to Psychological Therapies Data Set will be in included in a future version of the Mental Health Services Data Set.
Improving Access to Psychological Therapies Data Set Overview
Due to the rapidly changing situation with Covid-19 for both providers and NHS Digital, the transition from Improving Access to Psychological Therapies (IAPT) Data Set v1.5 to v2.0 has been postponed until 1 July 2020.The situation will be reviewed regularly and any decision regarding a further extension will be made at least two weeks prior to the new go-live date.
For further information please contact: enquiries@nhsdigital.nhs.uk.
Version 1.5 of the data set can be found at: IAPT Data Set.
For a "Full Screen" view, click Improving Access to Psychological Therapies Data Set.
In the "Full Screen" view, to return to the "Data Set" view, click the browser "back" button.
The Mandatory or Required (M/R/O) column indicates the recommendation for the inclusion of data.
- M = Mandatory: this data element is mandatory and the technical process (e.g. submission of the data set, production of output etc) cannot be completed without this data element being present
- R = Required: NHS business processes cannot be delivered without this data element
- O = Optional: the inclusion of this data element is optional as required for local purposes.
For guidance on the Data Set constraints, see the Improving Access to Psychological Therapies Data Set Constraints.
| HEADER |
|---|
| Header: To carry header details for the submission. One occurrence of this group is required. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | DATA SET VERSION NUMBER |
| M | ORGANISATION IDENTIFIER (CODE OF PROVIDER) |
| M | ORGANISATION IDENTIFIER (CODE OF SUBMITTING ORGANISATION) |
| M | PRIMARY DATA COLLECTION SYSTEM IN USE |
| M | REPORTING PERIOD START DATE |
| M | REPORTING PERIOD END DATE |
| M | DATE AND TIME DATA SET CREATED |
| PATIENT DEMOGRAPHICS |
|---|
| Master Patient Index: To carry personal details of the patient. One occurrence of this group is required. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| M | ORGANISATION IDENTIFIER (LOCAL PATIENT IDENTIFIER) |
| R | ORGANISATION IDENTIFIER (RESIDENCE RESPONSIBILITY) |
| R | NHS NUMBER |
| R | NHS NUMBER STATUS INDICATOR CODE |
| R | PERSON BIRTH DATE |
| R | POSTCODE OF USUAL ADDRESS |
| R | PERSON STATED GENDER CODE |
| R | ETHNIC CATEGORY |
| R | EX-BRITISH ARMED FORCES INDICATOR |
| R | LANGUAGE CODE (PREFERRED) |
| R | EDUCATIONAL ESTABLISHMENT TYPE (IMPROVING ACCESS TO PSYCHOLOGICAL THERAPIES) |
| GP Practice Registration: To carry details of the GP Practice Registration of the patient. One occurrence of this group is required for each change of GP Practice Registration. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| M | GENERAL MEDICAL PRACTICE CODE (PATIENT REGISTRATION) |
| R | START DATE (GMP PATIENT REGISTRATION) |
| R | END DATE (GMP PATIENT REGISTRATION) |
| R | ORGANISATION IDENTIFIER (GP PRACTICE RESPONSIBILITY) |
| Employment Status: To carry details of the employment status of the patient. One occurrence of this group is permitted for each employment status. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| M | EMPLOYMENT STATUS |
| R | EMPLOYMENT STATUS RECORDED DATE |
| R | WEEKLY HOURS WORKED |
| R | SELF EMPLOYED INDICATOR |
| R | SICKNESS ABSENCE INDICATOR |
| R | STATUTORY SICK PAY RECEIPT INDICATOR |
| R | BENEFIT RECEIPT INDICATOR (IMPROVING ACCESS TO PSYCHOLOGICAL THERAPIES) |
| R | JOBSEEKERS ALLOWANCE RECEIPT INDICATOR |
| R | EMPLOYMENT AND SUPPORT ALLOWANCE RECEIPT INDICATOR |
| R | UNIVERSAL CREDIT RECEIPT INDICATOR |
| R | PERSONAL INDEPENDENCE PAYMENT RECEIPT INDICATOR |
| R | OTHER BENEFITS RECEIPT INDICATOR (IMPROVING ACCESS TO PSYCHOLOGICAL THERAPIES) |
| R | EMPLOYMENT SUPPORT SUITABILITY INDICATOR |
| R | EMPLOYMENT SUPPORT REFERRAL DATE |
| Disability Type: To carry details of the type of disability affecting a patient, based on formal diagnoses, the patient’s perception or the perception of a patient proxy. One occurrence of this group is permitted for each disability identified. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| M | DISABILITY CODE |
| Social and Personal Circumstances To carry details of social and personal circumstances of a patient. One occurrence of this group is permitted for each social and personal circumstance recorded. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| M | SOCIAL AND PERSONAL CIRCUMSTANCE (SNOMED CT) |
| R | SOCIAL AND PERSONAL CIRCUMSTANCE RECORDED DATE |
| Overseas Visitor Charging Category To carry details of the Overseas Visitor Charging Category of the patient. Multiple occurrences of this group are permitted, one for each Overseas Visitor Charging Category recorded for the patient. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| M | OVERSEAS VISITOR CHARGING CATEGORY |
| R | OVERSEAS VISITOR CHARGING CATEGORY APPLICABLE DATE |
| REFERRALS |
|---|
| Service or Team Referral: To carry details of the Service or Team referral that the patient is subject to. One occurrence of this group is required for each referral. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | SERVICE REQUEST IDENTIFIER |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| M | ORGANISATION IDENTIFIER (CODE OF COMMISSIONER) |
| M | REFERRAL REQUEST RECEIVED DATE |
| R | SOURCE OF REFERRAL FOR MENTAL HEALTH |
| R | YEAR AND MONTH OF SYMPTOMS ONSET (IMPROVING ACCESS TO PSYCHOLOGICAL THERAPIES) |
| R | PREVIOUS DIAGNOSED CONDITION INDICATOR |
| R | DISCHARGE FROM IMPROVING ACCESS TO PSYCHOLOGICAL THERAPIES SERVICE REASON |
| R | SERVICE DISCHARGE DATE |
| Onward Referral: To carry details of any onward referral of the patient which has taken place. One occurrence of this group is permitted for each onward referral. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | SERVICE REQUEST IDENTIFIER |
| M | ONWARD REFERRAL DATE |
| R | ONWARD REFERRAL TIME |
| R | ONWARD REFERRAL REASON |
| R | ORGANISATION IDENTIFIER (RECEIVING) |
| WAITING TIME PAUSES |
|---|
| Waiting Time Pauses: To carry details of the Waiting Time Pauses. One occurrence is permitted for each Waiting Time Pause. | |
|---|---|
| M/R | Data Set Data Elements |
| M | IMPROVING ACCESS TO PSYCHOLOGICAL THERAPIES ACTIVITY SUSPENSION IDENTIFIER |
| M | SERVICE REQUEST IDENTIFIER |
| M | ACTIVITY SUSPENSION START DATE |
| R | ACTIVITY SUSPENSION END DATE |
| R | IMPROVING ACCESS TO PSYCHOLOGICAL THERAPIES ACTIVITY SUSPENSION REASON |
| CARE CONTACT, CARE ACTIVITIES AND INDIRECT ACTIVITIES |
|---|
| Care Activity: To carry details of any activities which have taken place as part of a Care Contact. One occurrence of this group is permitted for each Care Activity. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | CARE ACTIVITY IDENTIFIER |
| M | CARE CONTACT IDENTIFIER |
| R | CARE PERSONNEL LOCAL IDENTIFIER |
| R | CLINICAL CONTACT DURATION OF CARE ACTIVITY |
| R | CODED PROCEDURE AND PROCEDURE STATUS (SNOMED CT) |
| R | FINDING SCHEME IN USE |
| R | CODED FINDING (CODED CLINICAL ENTRY) |
| R | CODED OBSERVATION (SNOMED CT) |
| R | OBSERVATION VALUE |
| R | UCUM UNIT OF MEASUREMENT |
| Internet Enabled Therapy Care Professional Activity Log: To carry details of the summarised activity during a specified time period for the Care Professional supporting Internet Enabled Therapy for a patient. One occurrence this group is permitted for each activity log. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | SERVICE REQUEST IDENTIFIER |
| M | START DATE (INTERNET ENABLED THERAPY ACTIVITY LOG) |
| M | END DATE (INTERNET ENABLED THERAPY ACTIVITY LOG) |
| M | INTERNET ENABLED THERAPY PROGRAMME |
| M | DURATION OF INTERNET ENABLED THERAPY IMPROVING ACCESS TO PSYCHOLOGICAL THERAPIES CARE PROFESSIONAL CLINICAL TIME |
| R | CARE PERSONNEL LOCAL IDENTIFIER |
| R | INTERNET ENABLED THERAPY INTEGRATED SOFTWARE ENGINE USED INDICATOR |
| CLINICALLY CODED TERMINOLOGY |
|---|
| Long Term Physical Health Condition: To carry details of any Long Term Physical Health Conditions for a patient which are stated by the patient or recorded in medical notes One occurrence of this group is permitted for each Long Term Physical Health Condition. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | SERVICE REQUEST IDENTIFIER |
| M | FINDING SCHEME IN USE |
| M | LONG TERM PHYSICAL HEALTH CONDITION (CODED CLINICAL ENTRY) |
| Presenting Complaints: To carry details of the primary and any secondary presenting complaints recorded for a patient, made by the service that the patient was referred or admitted to. One occurrence of this group is permitted for each presenting complaint. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | SERVICE REQUEST IDENTIFIER |
| M | FINDING SCHEME IN USE |
| M | PRESENTING COMPLAINT (CODED CLINICAL ENTRY) |
| R | PRESENTING COMPLAINT CODING SIGNIFICANCE |
| R | PRESENTING COMPLAINT RECORDED DATE |
| Coded Scored Assessment (Referral): To carry details of scored assessments that are issued and completed as part of a Service Request, but do not take place at a specific contact. One occurrence of this group is permitted for each coded scored assessment question or dimension captured outside of a Care Contact. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | SERVICE REQUEST IDENTIFIER |
| M | CODED ASSESSMENT TOOL TYPE (SNOMED CT) |
| M | PERSON SCORE |
| M | ASSESSMENT TOOL COMPLETION DATE |
| R | ASSESSMENT TOOL COMPLETION TIME |
| Coded Scored Assessment (Care Activity): To carry details of scored assessments that are issued and completed as part of a specific Care Activity. One occurrence of this group is permitted for each coded scored assessment question or dimension captured as part of a specific Care Activity. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | CARE ACTIVITY IDENTIFIER |
| M | CODED ASSESSMENT TOOL TYPE (SNOMED CT) |
| M | PERSON SCORE |
| CARE CLUSTERS |
|---|
| Care Cluster: To carry details of the Care Cluster resulting from a clustering tool assessment. One occurrence of this group is permitted for each period of time that a patient was allocated to a Care Cluster. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | LOCAL PATIENT IDENTIFIER (EXTENDED) |
| M | ADULT MENTAL HEALTH CARE CLUSTER CODE (FINAL) |
| M | START DATE (CARE CLUSTER ASSIGNMENT PERIOD) |
| R | START TIME (CARE CLUSTER ASSIGNMENT PERIOD) |
| R | END DATE (CARE CLUSTER ASSIGNMENT PERIOD) |
| R | END TIME (CARE CLUSTER ASSIGNMENT PERIOD) |
| CARE PERSONNEL QUALIFICATION |
|---|
| Care Personnel: To carry details of each qualification attained or planned to be attained by the Care Personnel. One occurrence of this group is permitted for each qualification. | |
|---|---|
| M/R/O | Data Set Data Elements |
| M | CARE PERSONNEL LOCAL IDENTIFIER |
| M | QUALIFICATION ATTAINMENT LEVEL (IMPROVING ACCESS TO PSYCHOLOGICAL THERAPIES) |
| R | EMPLOYEE QUALIFICATION AWARDED DATE |
| R | EMPLOYEE QUALIFICATION PLANNED COMPLETION DATE |
Change to Supporting Information: Changed Description
An Adult Mental Health Care Cluster is a type of CARE CLUSTER for adult PATIENTS.
An Adult Mental Health Care Cluster is part of a currency developed to support the National Tariff Payment System for Mental Health Services.
Adult Mental Health Care Clusters are 21 groupings of Mental Health PATIENTS based on their characteristics, and are a way of classifying individuals utilising Mental Health Services that forms the basis for payment.
An Adult Mental Health Care Cluster is assigned using a decision tree or algorithm based on the PERSON SCORE from the Adult Mental Health Clustering Tool undertaken by a CARE PROFESSIONAL for the PATIENT.
This is done by first assigning the PATIENT to one of three Mental Health Care Cluster Super Classes, to narrow down the number of possible Adult Mental Health Care Clusters which are applicable to the PATIENTS condition. The PATIENT is then assigned to the most appropriate of this sub-set of Adult Mental Health Care Clusters.
Further information relating to the Adult Mental Health Clustering Tool and Adult Mental Health Care Clusters is available from the gov.uk website at: The NHS payment system: documents and guidance.uk website at: The NHS payment system: documents and guidance.
Change to Supporting Information: Changed Description
The Adult Mental Health Clustering Tool is a type of Clustering Tool for adult PATIENTS receiving Mental Health care.
The Adult Mental Health Clustering Tool is a needs assessment tool designed to rate the care needs of a PATIENT, based upon a series of 18 rating scales.
The first 12 of these rating scales are the same as the Health of the Nation Outcome Scale (Working Age Adults) rating scales, originally developed by the Royal College of Psychiatrists. These 12 rating scales are numbered 1 - 12 under 'Current Ratings' in the Adult Mental Health Clustering Tool.
One additional 'current' rating and a new section relating to historical ratings have also been added, to form the Adult Mental Health Clustering Tool. These items are referred to as the Summary Assessment of Characteristics (SAC) items.
Part 1: Current Ratings
These ratings relate to the most severe occurrence in the two weeks prior to the Adult Mental Health Clustering Tool ASSESSMENT TOOL COMPLETION DATE.
- 1. Overactive, aggressive, disruptive or agitated behaviour (current)
- 2. Non-accidental self injury (current)
- 3. Problem drinking or drug taking (current)
- 4. Cognitive problems (current)
- 5. Physical illness or disability problems (current)
- 6. Problems associated with hallucinations and delusions (current)
- 7. Problems with depressed mood (current)
- 8. Other mental and behavioural problems (current), qualified by specific disorders: and the alphabetical list of headings from the glossary:
- A. Phobic
- B. Anxiety
- C. Obsessive-compulsive
- D. Mental strain/tension
- E. Dissociative
- F. Somatoform
- G. Eating
- H. Sleep
- I. Sexual
- J. Other
- 9. Problems with relationships (current)
- 10. Problems with activities of daily living (current)
- 11. Problems with living conditions (current)
- 12. Problems with occupation and activities (current)
- 13. Strong unreasonable beliefs that are not psychotic in origin (current)
Part 2: Historical Ratings
These ratings relate to problems that occur in an episodic or unpredictable way, from a more 'historical' perspective. Whilst there may not be any direct observation or report of a manifestation during the two weeks prior to the Adult Mental Health Clustering Tool ASSESSMENT TOOL COMPLETION DATE, the evidence and clinical judgement would suggest that there is still a cause for concern that cannot be disregarded. In these circumstances, any event that remains relevant to the current CARE PLAN should be included.
- A. Agitated behaviour / expansive mood (historical)
- B. Repeat self-harm (historical)
- C. Safeguarding other children and vulnerable adults (historical)
- D. Engagement (historical)
- E. Vulnerability (historical)
The allowed responses to each of the 18 items in the Adult Mental Health Clustering Tool are:
- 0 - No problem
- 1 - Minor problem requiring no action
- 2 - Mild problem but definitely present
- 3 - Moderately severe problem
- 4 - Severe to very severe problem
- 9 - Not known
The PERSON SCORE from the Adult Mental Health Clustering Tool is used to allocate the PATIENT to the most appropriate Adult Mental Health Care Cluster.
Further information relating to the Adult Mental Health Clustering Tool and Adult Mental Health Care Clusters is available from the gov.uk website at:
Change to Supporting Information: Changed Description
A Forensic Mental Health Care Cluster is a type of CARE CLUSTER for PATIENTS accessing Forensic Mental Health Services.
A Forensic Mental Health Care Cluster is part of a currency developed to support the National Tariff Payment System for Forensic Mental Health Services.
Forensic Mental Health Care Clusters are 22 groupings of Forensic Mental Health Patients based on their characteristics.
A Forensic Mental Health Care Cluster is assigned using a decision tree or algorithm based on the PERSON SCORE from the Forensic Mental Health Clustering Tool undertaken by a CARE PROFESSIONAL for the PATIENT.
This is done by first assigning the PATIENT to one of three Mental Health Care Cluster Super Classes, to narrow down the number of possible Forensic Mental Health Care Clusters which are applicable to the PATIENT's condition. The PATIENT is then assigned to the most appropriate of this sub-set of Forensic Mental Health Care Clusters.
Further information relating to the Forensic Mental Health Clustering Tool and Forensic Mental Health Care Cluster is available from the gov.uk website at: The NHS payment system: documents and guidance.uk website at: The NHS payment system: documents and guidance.
Change to Supporting Information: Changed Description
The Market Forces Factor (MFF) is an estimate of unavoidable cost differences between Health Care Providers, based on their geographical location.
The Market Forces Factor is used to adjust resource allocations in the NHS in proportion to these cost differences, so that PATIENTS are neither advantaged nor disadvantaged by the relative level of unavoidable costs in different parts of the country.
For further information on the Market Forces Factor, see the NHS Improvement website at: Guidance on the market forces factor.For further information on the Market Forces Factor, see the NHS Improvement website at: A guide to the market forces factor.
Change to Supporting Information: Changed Description
A Mental Health Care Cluster Super Class is identified during the process of assigning an Adult Mental Health Care Cluster or Forensic Mental Health Care Cluster to a PATIENT.
A Mental Health Care Cluster Super Class enables the number of applicable Adult Mental Health Care Clusters or Forensic Mental Health Care Clusters to be narrowed down, by deciding if the origin of the presenting condition is primarily:
- Non-psychotic
- Psychotic or
- Organic
If the PATIENT cannot be assigned to an Adult Mental Health Care Cluster or Forensic Mental Health Care Cluster, MENTAL HEALTH CARE CLUSTER SUPER CLASS CODE is recorded as 'Unable to assign PATIENT to Mental Health Care Cluster Super Class'. The PATIENT will automatically be assigned to the ADULT MENTAL HEALTH CARE CLUSTER CODE or FORENSIC MENTAL HEALTH CARE CLUSTER CODE '00 Care Cluster 0 - Variance.'
Further information relating to the Adult Mental Health Clustering Tool and Adult Mental Health Care Clusters is available from the gov.uk website at: The NHS payment system: documents and guidance.uk website at: The NHS payment system: documents and guidance.
Change to Supporting Information: Changed Description
The National Tariff Payment System is managed by NHS England and NHS Improvement.
The National Tariff Payment System sets out the national tariff for each year.
This set of prices and rules helps local Clinical Commissioning Groups work with Health Care Providers, such as NHS Trusts and NHS Foundation Trusts to identify which health care SERVICES provide best value to their PATIENTS.
For further information on the National Tariff Payment System, see the:
- NHS England website at: NHS payment system
gov.uk website at:The NHS payment system: documents and guidance.- gov.uk website at: The NHS payment system: documents and guidance.
Change to Supporting Information: Changed Description
Safeguarding Children is a SAFEGUARDING CHILDREN OBSERVATION.
The term "Safeguarding Children" is defined as:
The process of protecting children from abuse or neglect, preventing impairment of their health and development, and ensuring they are growing up in circumstances consistent with the provision of safe and effective care that enables children to have optimum life chances and enter adulthood successfully.
For further information, see the "Schools, colleges and children's services" part of the gov.uk website at: Safeguarding children.
Change to Supporting Information: Changed Description
A Social Care Worker is a PERSON.
Social Care Workers provide the practical support to help people cope with the day-to-day business of living.
Social Care Workers may be home care assistants or work in residential care homes. There are a wide range of jobs working with older people, children and families and people with disabilities.
For further information on Social Care Workers, see the Skills for Care website.
Change to Supporting Information: Changed Description
A Social Worker is a CARE PROFESSIONAL.
The social work profession promotes social change, problem solving in human relationships, protection on behalf of the public, and the empowerment and liberation of people to enhance wellbeing.
Social Workers form partnerships with people to help them identify and interpret any problems they face and to help them to find solutions. The profession is graduate entry and Social Workers work with legal structures and with a range of other professionals.
In England only an individual registered with the Health and Care Professions Council (HCPC) is legally able to work as a Social Worker.
For further information on Social Workers, see the British Association of Social Workers website.
Change to Supporting Information: Changed Description, Name
Information Standards Notices and Data Dictionary Change Notices incorporated into the NHS Data Model and Dictionary:
April 2020
- CR1667 (Immediate) - DCB1077 AIDC for Patient Identification Data Set Update
- CR1760 (1 April 2021) - DCB0028 Main Specialty Code and Treatment Function Code Updates
- Note: New National Codes were introduced from 2 April 2020 and should not be reported nationally until the functionality to do so becomes available in the next release of the affected data sets. A note has been added to the affected items to provide further guidance.
March 2020
- CR1690 (1 April 2020) - DCB1069 Community Services Data Set Version 1.5
- CR1714 (1 April 2020) - DCB1521 Cancer Outcomes and Services Data Set Version 9
- CR1719 (1 April 2020) - DCB1520 Improving Access to Psychological Therapies Data Set Version 2
- CR1732 (1 April 2020) - DCB0011 Mental Health Services Data Set Version 4.1
- CR1723 (1 April 2020) - DCB0084 Introduction of OPCS-4.9
- CR1743 (1 April 2020) - DCB1533 Aggregate Contract Monitoring Data Set Update
- CR1744 (1 April 2020) - DCB1533 Patient Level Contract Monitoring Data Set Update
- CR1753 (1 April 2020) - DCB0089 Cover of Vaccination Evaluated Rapidly (COVER) Central Return Data Set Update
- CR1757 (1 April 2020) - DDCN 1757/2020 Organisation Data Service Information Update
- CR1752 (Immediate) - DDCN 1752/2020 Practitioners with a Special Interest Name Change
Release: December 2019
- CR1746 (Immediate) - DCB0039 GUMCAD Sexually Transmitted Infection Surveillance System Data Set
- CR1747 (Immediate) - DCB2212 Drugs Patient Level Contract Monitoring Data Set Update
- CR1724 (1 January 2020) - DCB1533 National Workforce Data Set Version 3.1
- CR1756 (Immediate) - DDCN 1756/2019 Social Work England
- CR1703 (Immediate) - DDCN 1703/2019 Update to GP Default Code V81998 Definition
- CR1741 (Immediate) - DDCN 1741/2019 Critical Care Minimum Data Set
Release: October 2019
- CR1668 (Immediate) - DDCN 1668/2019 Retirement of National Renal Data Set
Release: August 2019
- CR1661 (1 September 2019) - DCB1533 Systemic Anti-Cancer Therapy Data Set Version 3
- CR1734 (Immediate) - DDCN 1734/2019 Consultation Medium Used Update
- CR1731 (Immediate) - DDCN 1731/2019 Retirement of Quarterly Bed Availability and Occupancy Data Set (KH03)
- CR1735 (Immediate) - DDCN 1735/2019 Retirement of Mixed Sex Accommodation Data Set
- CR1641 (Immediate) - DDCN 1641/2019 Retirement of Emergency Care Weekly Situation Report Data Set
Release: July 2019
- CR1634 (Immediate) - DCB2123 Patient Level Information Costing System (PLICS) Acute Data Set
- CR1696 (Immediate) - DDCN 1696/2019 SNOMED CT Subsets
- CR1722 (Immediate) - DDCN 1722/2019 GUMCAD Sexually Transmitted Infection Surveillance System Data Set
Release: May 2019
- CR1611 (Immediate) - DCB2050 Introduction of the Aggregate Contract Monitoring Data Set
- CR1613 (Immediate) - DCB3003 Introduction of Patient Level Contract Monitoring Data Set
- CR1612 (Immediate) - DCB3002 Introduction of Devices Patient Level Contract Monitoring Data Set
- CR1615 (Immediate) - DCB2212 Introduction of Drugs Patient Level Contract Monitoring Data Set
- CR1713 (Immediate) - DCB1593 Venous Thromboembolism Risk Assessment Data Set Update
- CR1730 (Immediate) - DDCN 1730/2019 Retirement of Diagnostics Waiting Times and Activity Data Set
- CR1729 (Immediate) - DDCN 1729/2019 Retirement of Diagnostics Waiting Times Census Data Set
- CR1728 (Immediate) - DDCN 1728/2019 Retirement of Referral To Treatment Data Set
- CR1727 (Immediate) - DDCN 1727/2019 Retirement of National Direct Access Audiology Data Set
- CR1725 (Immediate) - DDCN 1725/2019 Retirement of Quarterly Monitoring Cancelled Operations Data Set (QMCO)
Release: April 2019
- CR1704 (1 April 2019) - DCB0089 Cover of Vaccination Evaluated Rapidly (COVER) Central Return Data Set Update
- CR1708 (Immediate) - DDCN 1708/2019 Retirement of KC50: Immunisation Programmes Activity Data Set (KC50)
- CR1700 (Immediate) - DDCN 1700/2019 Retirement of KC65: Colposcopy Clinics: Referrals, Treatments and Outcomes
- CR1699 (Immediate) - DDCN 1699/2019 Retirement of KC53: Adult Screening Programmes - Cervical Screening
- CR1698 (Immediate) - DDCN 1698/2019 Retirement of Edifact Items
- CR1568 (Immediate) - DDCN 1568/2019 Retirement of KC61: Pathology Laboratories: Cervical Screening and Outcome of Referrals
- CR1536 (Immediate) - DDCN 1536/2019 Retirement of KH12: Imaging and Radiological Examinations or Tests in any Part of a Hospital
- CR1499 (Immediate) - DDCN 1499/2019 Retirement of NHS Health Checks Data Set
Release: March 2019
- CR1550 (1 April 2019) - DCB1513 Maternity Services Data Set Version 2
- CR1648 (1 April 2019) - DCB0011 Mental Health Services Data Set Version 4
- CR1654 (1 April 2019) - DCB0092-2062 Update to Commissioning Data Set Type 011 Emergency Care
- CR1715 (1 April 2019) - DCB0092-2062 Corrigendum to Commissioning Data Set V6-2-2 Type 011 Emergency Care
- CR1717 (1 April 2019) - DCB0092-2062 Corrigendum to Commissioning Data Set V6-2-1 Type 011 Emergency Care
- CR1695 (Immediate) - DDCN 1695/2019 Emergency Care Department Type - Urgent Treatment Centres
- CR1704 (1 April 2019) - DCB0089 Cover of Vaccination Evaluated Rapidly (COVER) Central Return Data Set Update
Release: December 2018
- CR1650 (1 January 2018) - DCB1067 National Workforce Data Set v3.0
Release: November 2018
- CR1631 (Immediate) - DCB3017 Overseas Visitor Charging Category
Release: October 2018
- CR1618 (1 October 2018) - DCB0039 GUMCAD Sexually Transmitted Infection Surveillance System Data Set
- CR1666 (Immediate) - DDCN 1666/2018 Stop Smoking Services Quarterly Data Set
Release: September 2018
- CR1656 (Immediate) - DDCN 1656/2018 NHS Data Model Update
- CR1665 (Immediate) - DDCN 1665/2018 Person Marital Status
- CR1645 (Immediate) - DDCN 1645/2018 Specialised Commissioning: Removal of Default Code YDD82
Release: July 2018
- CR1639 (1 April 2018) - DCB2117 NHS Continuing Healthcare Data Set Update
- CR1630 (4 June 2018) - DCB1567 National Joint Registry Data Set Version 7
- CR1658 (Immediate) - DDCN 1658/2018 Young Offender Institution
Release: May 2018
- CR1653 (Immediate) - DDCN 1653/2018 HIV and AIDS Reporting Data Set Update
- CR1647 (Immediate) - DDCN 1647/2018 Terminology and Classifications Update
Release: April 2018
- CR1636 (Immediate) - DDCN 1636/2018 Department of Health: Change of name
- CR1638 (30 April 2018) - DCB0090 Health and Social Care Organisation Reference Data: Introducing an Application Programming Interface (API)
Release: March 2018
- CR1588 (1 April 2018) - DCB1521 Cancer Outcomes and Services Data Set Version 8
- CR1589 (1 April 2018) - DCB0147 National Cancer Waiting Times Monitoring Data Set Version 2.0
- CR1610 (1 April 2018) - DCB0011 Mental Health Services Data Set Version 3.0
- CR1642 (Immediate) - DDCN 1642/2018 Youth Offenders Institute: Change of name
- CR1635 (Immediate) - DDCN 1635/2018 Edubase: Change of name
Release: January 2018
- CR1633 (Immediate) - DDCN 1633/2017 Introduction of Strategic Data Collection Service (SDCS) and Data Services Platform (DSP)
Release: November 2017
- CR1623 (Immediate) - DCB0089 Cover of Vaccination Evaluated Rapidly (COVER) Central Return Data Set
- CR1628 (Immediate) - DDCN 1628/2017 Employment Status
Release: October 2017
- CR1619 (1 October 2017) - DCB1067 National Workforce Data Set Version 2.9
- CR1595 (Immediate) - DCB0155 Stop Smoking Services Quarterly Data Set
- CR1572 (Immediate) - DCB2094 Sexual Orientation
- The October 2017 Release updates the NHS Data Model and Dictionary Help Pages and Demonstrations to reflect the new organisation structure.
Release: September 2017
- CR1587 (1 October 2017) - SCCI1069 Community Services Data Set (CSDS) Version 1.0
Release: July 2017
- CR1555 (Immediate) - SCCI1518 Sexual and Reproductive Health Activity Data Set Changes
- CR1606 (Immediate) - SCCI0011 Mental Health Services Data Set Version 2 Corrigendum
Release: June 2017
- CR1607 (Immediate) - DDCN 1607/2017 Renaming of NHS Commissioning Board Commissioning Region and NHS England Region (Geography)
- CR1604 (Immediate) - DDCN 1604/2017 Introduction of the Data Coordination Board
Release: April 2017
The following has been incorporated early to allow users to see the changes, but please note that the implementation date is 1 October 2017:
- CR1598 (1 October 2017) - SCCI0092-2062 Commissioning Data Set Type 011 Emergency Care
Release: March 2017
- CR1605 (Immediate) - DDCN 1605/2017 NHS Number Status Indicator Code
- CR1594 (Immediate) - DDCN 1594/2017 Technology Reference Data Update Distribution (TRUD)
- CR1564 (01 April 2017) - SCCI1521 Cancer Outcomes and Services Data Set Version 7
- CR1563 (01 April 2017) - SCCI0011 Mental Health Services Data Set Version 2.0
- CR1577 (01 April 2017) - SCCI0084 Introduction of OPCS-4.8
Release: December 2016
- CR1601 (Immediate) - DDCN 1601/2016 Organisation Identifier Data Elements
- CR1600 (Immediate) - DDCN 1600/2016 HES Data Dictionary
Release: November 2016
- CR1442 (01 November 2016) - SCCI1570 Amd 20/2015 HIV and AIDS Reporting Data Set and XML Schema Version 3
- CR1531 (Immediate) - DDCN 1531/2016 Clinical Terminology Update
Release: October 2016
- CR1578 (Immediate) - DDCN 1578/2016 Religious or Other Belief System Affiliation Groups SNOMED CT Subset
- CR1569 (Immediate) - DDCN 1569/2016 NHS Improvement
Release: September 2016
- CR1545 (Immediate) - SCCI0075 and SCCI0076 Updates to the Neonatal Critical Care and Paediatric Critical Care Minimum Data Sets
Release: August 2016
- CR1532 (Immediate) - SCCI0090 Health and Social Care Organisation Reference Data
- CR1583 (Immediate) - DDCN 1583/2016 Introduction of NHS Digital
- CR1575 (Immediate) - DDCN 1575/2016 Introduction of the National Cancer Registration and Analysis Service (NCRAS)
- CR1570 (Immediate) - DDCN 1570/2016 Update to COVER Central Return Data Set
Release: July 2016
- CR1565 (Immediate) - ISB 1561 Retirement of Diabetes Summary Core Data Set ISB 1561
Release: March 2016
- CR1300 (1 April 2016) - SCCI01477 Updates to the National Cancer Waiting Times Monitoring Data Set and introduction of the XML Schema
- CR1412 (1 April 2016) - SCCI0021 Introduction of the International Classification of Diseases (ICD) 10th Revision 5th Edition
- CR1544 (1 April 2016) - SCCI1111 Radiotherapy Data Set - Change of data flow
- CR1549 (1 April 2016) - SCCII0011 Mental Health Services Data Set Version 1.1
Release: February 2016
- CR1517 (1 January 2016) - SCCI1067 Workforce Data Set Version 2.8
- CR1559 (Immediate) - DDCN 1559/2016 Lower Layer Super Output Area (Residence) and ONS Local Government Geography Code (Local Authority District)
Release: December 2015
- CR1514 (1 January 2016) - SCCI0011 Mental Health Services Data Set
- CR1515 (1 January 2016) - SCCI0011 Retirement of Mental Health Standards
- CR1560 (Immediate) - DDCN 1560/2015 Retirement of Data Management and Integration Centre
Release: November 2015
- CR1558 (Immediate) - DDCN 1558/2015 Children and Young People’s Health Services Data Set and Community Information Data Set Inconsistencies
- CR1554 (1 October 2015) - SCCI2026 Corrigendum to CR1494 Female Genital Mutilation Data Set
Release: October 2015
- CR1534 (Immediate) - DDCN 1534/2015 Retirement of Hospital Episode Statistics Cross Reference Tables
Release: September 2015
- CR1521 (Immediate) - SCCI1580 Palliative Care Co-ordination: Core content (Formerly End of Life Care)
- CR1522 (Immediate) - DDCN 1522/2015 Update General Dental Council Registration Number
- CR1530 (Immediate) - ISB 0158 Retirement of Ambulance Services (KA34) Central Return Data Set
- CR1528 (Immediate) - ISB 1568 Retirement of KO41 (A) Hospital and Community Health Service Complaints and KO41 (B) General Practice (including Dental) Complaints Central Return Forms
- CR1551 (Immediate) - ISB 0133 Retirement of HPV Immunisation Programme Vaccine Monitoring Monthly Minimum Data Set and HPV Immunisation Programme Vaccine Monitoring Annual Minimum Data Set
Release: August 2015
- CR1374 (1 September 2015) - SCCI1510 Community Information Data Set Update
- CR1356 (1 September 2015) - SCCI1069 Children and Young People’s Health Services Data Set Update and XML Schema
- CR1529 (Immediate) - DDCN 1529/2015 Change to the Mechanism for XML Schema Publication and Download
- CR1543 (Immediate) - DDCN 1543/2015 Treatment Function Code: 840 Audiology
Release: July 2015
- CR1475 (Immediate) - SCCI1605 Accessible Information
Release: June 2015
- CR1518 (Immediate) - ISB 092 CDS 6-1 Retirement
- CR1525 (Immediate) - DDCN 1525/2015 Burden Advice and Assessment Service (BAAS)
- CR1524 (Immediate) - DDCN 1524/2015 Updating of Activity Location Type and Source of Admission Attributes
- CR1505 (Immediate) - DDCN 1505/2015 Death Cause Information
Release: May 2015
- CR1507 (Immediate) - DDCN 1507/2015 To add SUS CDS business rule H4 text
Release: April 2015
- CR 1494 and CR 1506 (1 April 2015) - SCCI2026 Amd 12/2014 Female Genital Mutilation Data Set and Retirement of Female Genital Mutilation Prevalence Data Set
- CR1513 (27 April 2015) - DDCN 1513/2015 Introduction of NHS England Region (Geography)
- CR1509 (1 April 2015) - ISB 1513 Maternity Services Data Set
CR1509 is a corrigendum to CR1355 (1 November 2014) - ISB 1513 Amd 45/2012 Maternity Services Data Set Update and XML Schema published in the October 2014 release
Release: March 2015
- CR1492 (1 April 2015) - SCCI1521 Amd 17/2014 Updates to the Cancer Outcomes and Services Data Set and XML Schema
Release: February 2015
- CR1486 (27 February 2015) - ISB 0090 Amd 9/2014 Organisation Data Service – Health and Justice Organisation Identifiers
Due to a delay in the Organisation Data Service (ODS) February release, the implementation date is now 6 March 2015.
Release: January 2015
- CR1473 (1 January 2015) - ISB 1538 Amd 13/2014 Chlamydia Testing Activity Data Set Update
- CR1496 (Immediate) - DDCN 1496/2015 Clinical Coding
Release: December 2014
- CR1396 (31 October 2014) - ISB 1567 Amd 15/2014 National Joint Registry Data Set Version 6
The following has been incorporated early to allow users to see the changes, but please note that the implementation date is 1 October 2015:
- CR1487 (1 October 2015) - ISB 0089 Amd 8/2014 Cover of Vaccination Evaluated Rapidly (COVER) Central Return Data Set
Release: November 2014
- CR1420 (Immediate) - ISB 0139 Amd 29/2013 Genitourinary Medicine Clinic Activity Data Set (GUMCAD) Update
- CR1421 (Immediate) - ISB 1518 Amd 30/2013 Sexual and Reproductive Health Activity Data Set (SRHAD) Update
- CR1422 (Immediate) - ISB 1518 Amd 30/2013 Retirement of Central Return Form KT31 Cross Sector Services
Release: October 2014
- CR1355 (1 November 2014) - ISB 1513 Amd 45/2012 Maternity Services Data Set Update and XML Schema
Release: September 2014
- CR1484 (Immediate) - DDCN 1484/2014 Female Genital Mutilation SNOMED CT Subsets
The following has been incorporated early to allow users to see the changes, but please note that the implementation date is 31 July 2015:
- CR1344 (31 July 2015) - ISB 1594 Amd 31/2012 Information Sharing to Tackle Violence Minimum Data Set
Release: August 2014
- CR1360 (1 September 2014) - ISB 0011 Amd 5/2014 Mental Health and Learning Disabilities Data Set
Release: July 2014
- CR1351 (1 July 2014) - ISB 1520 Amd 02/2013 Improving Access to Psychological Therapies Data Set Version 1.5
- CR1482 (Immediate) - DDCN 1482/2014 Source of Referral for Mental Health
- CR1480 (Immediate) - DDCN 1480/2014 Mental Health Care Cluster 9
- CR1477 (Immediate) - DDCN 1477/2014 Payment by Results
- Note: CR1383 (31 December 2014) - ISB 1555 Amd 10/2012 Personal Demographics Service Birth Notification Data Sets
At the Standardisation Committee for Care Information meeting on 28th May 2014, an amendment to the implementation date of the ISB information standard was approved. The implementation date is now 31 December 2014.
- The July 2014 Release updates the NHS Data Model and Dictionary Help Pages to reflect the new organisation structure.
Release: June 2014
- CR1465 (Immediate) - DDCN 1465/2014 Primary Care Trusts and NHS Trusts
- CR1461 (Immediate) - DDCN 1461/2014 New Standardisation Committee for Care Information (SCCI) Process
- CR1383 (30 June 2014) - ISB 1555 Amd 10/2012 Personal Demographics Service Birth Notification Data Sets
Release: May 2014
- CR1353 (1 June 2014) - ISB 1067 Amd 22/2013 Workforce Data Set Version 2.7
Release: April 2014
- CR1449 (Immediate) - ISB 1610 Amd 01/2014 Female Genital Mutilation Prevalence Data Set
Release: March 2014
- CR1388 (1 April 2014) - ISB 1521 Amd 23/2013 Updates to the Cancer Outcomes and Services Data Set and XML Schema
- CR1370 (1 April 2014) - ISB 1533 Amd 24/2013 Updates to the Systemic Anti-Cancer Therapy Data Set and XML Schema
- CR1322 (1 April 2014) - ISB 0111 Amd 26/2012 Changes to the Radiotherapy Data Set
- CR1387 (1 April 2014) - ISB 0084 Amd 10/2013 Introduction of OPCS-4.7
- CR1376 (1 April 2014) - ISB 1607 Amd 26/2013 Emergency Care Weekly Situation Report Data Set
- CR1433 (Immediate) - DDCN 1433/2014 Data Services for Commissioners
- CR1467 (1 April 2014) - DDCN 1467/2014 Retirement of Standards
- CR1464 (1 April 2014) - DDCN 1464/2014 Retirement of Standards - Domains and Diagrams
- CR1458 (1 April 2014) - DDCN 1458/2014 Retirement of Standards - DSCNs - 11/97/P05, 12/97/P06, 15/97/P09, 18/97/P12, 22/96/P19, 32/96/P27, 49/97/P35, 62/95/P51, 07/2007, 08/2009, 17/92, 20/2001, 22/2006 and 38/2002
- CR1444 (1 April 2014) - DDCN 1444/2014 Retirement of Standards
- CR1436 (1 April 2014) - DDCN 1436/2014 Retirement of Standards
- CR1435 (1 April 2014) - DDCN 1435/2014 Retirement of Standards - DSCNs 22/95/P21, 20/91, 21/93, 40/95/P34, 09/94/P04, 93/95/P76, 23/94/A04, 8/92 and 17/93
- CR1432 (1 April 2014) - DDCN 1432/2014 Retirement of Standards - DSCN 3/92, DSCN 12/96/P11, DSCN 50/94/P36, DSCN 66/96/W09 and DSCN 16/93
- CR1429 (1 April 2014) - DDCN 1429/2014 Retirement of Standards - DSCN 07/96/P06
- CR1425 (1 April 2014) - DDCN 1425/2014 Retirement of Standards
- CR1423 (1 April 2014) - DDCN 1423/2014 Retirement of Standards - DSCNs 37/98/A09, 14/97/P08, 12/2002, 37/2003, 14/2004 and 27/2001
- CR1419 (1 April 2014) - DDCN 1419/2014 Retirement of Standards - DSCNs 39/98/A11, 09/99/P06, 11/99/P07, 13/2003, 38/2001, 22/2001, 19/98/A02, 40/96/P34, 29/94/P19, 49/94/P35, 34/95/P29, 53/96/P44 and 96/95/P79
- CR1418 (1 April 2014) - DDCN 1418/2014 Retirement of Standards
- CR1417 (1 April 2014) - DDCN 1417/2014 Retirement of Standards - DSCNs 13/95/P12, 44/2001, 29/2004, 18/98/W02 and 24/98/F01
- CR1416 (1 April 2014) - DDCN 1416/2014 Retirement of Standards - KC64 - DSCNs 05/98/P05 and 26/95/W02
- CR1414 (1 April 2014) - DDCN 1414/2014 Retirement of Standards - DSCNs 03/99/P03, 10/2002, 12/99/A04, 20/98/A03, 30/98/P21, 35/99/P25, 37/97/P24 and 43/97/P29
- CR1413 (1 April 2014) - DDCN 1413/2014 Retirement of Standards - DSCNs 13/97/P07, 15/96/P14, 17/2001, 20/2004, 21/2001, 21/2003, 28/98/P20, 33/2003 and 43/2002
- CR1409 (1 April 2014) - DDCN 1409/2014 Retirement of Standards - DSCN's 46/97/P32, 01/2004, 04/2004, 11/2005, 27/2002, 31/2002, 53/2002 and 54/2002
Release: February 2014
- CR1460 (Immediate) - DDCN 1460/2014 NHS Dental Services Update
- CR1459 (Immediate) - DDCN 1459/2014 General Medical Practitioner (Specified), Doctor Index Number and General Medical Practitioner PPD Code Update
- CR1446 (Immediate) - DDCN 1446/2014 Health and Social Care Information Centre Update
- CR1404 (Immediate) - DDCN 1404/2014 Retirement of e-Gif definitions
- CR1395 (28 February 2014) - ISB 0090 Amd 17/2013 Organisation Data Service – NHS Postcode Directory
Release: January 2014
- CR1386 (31 January 2014) - ISB 0090 Amd 9/2013 Special Health Authority (SpHA) Code Structure Change
- CR1443 (Immediate) - DDCN 1443/2014 Change of name of the National Institute for Health and Clinical Excellence
- CR1441 (Immediate) - DDCN 1441/2014 Retirement of Review of Central Returns (ROCR) - Central Return Form KH03A
- CR1440 (Immediate) - DDCN 1440/2014 Retirement of Review of Central Returns (ROCR) - Genitourinary Medicine Access Monthly Monitoring Data Set
- CR1439 (Immediate) - DDCN 1439/2013 Retirement of Review of Central Returns (ROCR) Returns
- CR1405 (Immediate) - DDCN 1405/2013 Overseas Visitors
- CR1393 (Immediate) - DDCN 1393/2013 Amendment to Inter-Provider Transfer Administrative Minimum Data Set Overview
- CR1392 (Immediate) - DDCN 1392/2013 Review of Central Returns (ROCR) Discontinuations - Referral to Treatment Performance Sharing Data Set
- CR1391 (Immediate) - DDCN 1391/2013 Review of Central Returns (ROCR) Discontinuations - Referral to Treatment (RTT) Summary Patient Tracking List Data Set
The following have been incorporated early to allow users to see the changes, but please note that the implementation date is 1 June 2014:
- CR1346 (1 June 2014) - ISB 1595 Amd 32/2012 National Neonatal Data Set
Release: November 2013
- CR1424 (Immediate) - DDCN 1424/2013 Application Identifier (GS1)
- CR1367 (29 November 2013) - ISB 0090 Amd 5/2013 Organisation Data Service - Introduction of New Sub Type Identifier for Private Dental Practices
- CR1359 (29 November 2013) - ISB 0090 Amd 47/2012 Organisation Data Service - Identification Codes for Local Authorities
- CR1407 (Immediate) - DDCN 1407/2013 Clinical Investigations
- CR1415 (Immediate) - DDCN 1415/2013 Area Teams
- CR1411 (Immediate) - DDCN 1411/2013 Update to Supporting Information: SNOMED CT®
Release: September 2013
- CR1348 (1 October 2013) - ISB 1597 Amd 35/2012 Breast Screening Programmes Data Set (KC63 and KC62)
- CR1403 (Immediate) - DDCN 1403/2013 Religious or Other Belief System Affiliation
- CR1384 (Immediate) - DDCN 1384/2013 Health and Social Care Information Centre Rebranding of XML Schemas
- CR1397 (Immediate) - DDCN 1397/2013 Retired Main Specialty Codes
Release: July 2013
- CR1377 (Immediate) - ISB 0105 Retirement of Accident and Emergency Quarterly Monitoring Data Set (QMAE)
Release: May 2013
- CR1363 (Immediate) - ISB 1067 Amd 43/2012 National Workforce Data Set Version 2.6
- CR1382 (Immediate) - DDCN 1382/2013 National Renal Data Set amendment
- CR1381 (Immediate) - DDCN 1381/2013 Healthcare Resource Groups
- CR1235 (1 June 2013) - ISB 1588 Amd 11/2012 Accident and Emergency Clinical Quality Indicators
Release: April 2013
- CR1372 (Immediate) - DDCN 1372/2013 Organisation Update: April 2013
- CR1369 (Immediate) - DDCN 1369/2013 Organisation Codes and Organisation Types
- CR1347 (1 April 2013) - ISB 1521 Amd 40/2012 Updates to the Cancer Outcomes and Services Data Set and XML Schema
Release: March 2013
- CR1364 (Immediate) - DDCN 1364/2013 Operating Theatre
- CR1335 (1 April 2013) - ISB 1593 Amd 27/2012 Venous Thromboembolism Risk Assessment Data Set
- CR1340 (1 April 2013) - ISB 0090 Amd 37/2012 Organisation Data Service - Non-Legislative Organisations
- CR1321 (1 April 2013) - ISB 0011 Amd 25/2012 Mental Health Minimum Data Set version 4.1
Release: February 2013
- CR1336 (Immediate) - DDCN 1336/2013 XML Schema Constraint Pages
- CR1362 (Immediate) - DDCN 1362/2013 Update to Organisations in the NHS Data Model and Dictionary
- CR1246 (Immediate) - DDCN 1246/2013 Guidance for Merging Organisations
- CR1345 (Immediate) - DDCN 1345/2013 e-Government Interoperability Framework (e-GIF) and Government Data Standards Catalogue
- CR1354 (Immediate) - DDCN 1354/2013 Treatment Function Code - Well Babies
Release: December 2012
- CR1155 (Immediate) - ISB 1567 Amd 12/2011 National Joint Registry Data Set Version 5
- CR1324 (1 December 2012) - ISB 1067 Amd 23/2012 Workforce Data Set Version 2.5
- CR1196, CR1287 and CR1195 (1 January 2013) - ISB 1521 Amd 64/2010 Cancer Outcomes and Services Data Set, Cancer Outcomes and Services Data Set Message and Retirement of Cancer Registration Data Set and National Cancer Data Set
The following have been incorporated early to allow users to see the changes, but please note that the implementation date is 1 April 2013:
- CR1337 (1 April 2013) - ISB 1072 Amd 30/2012 Update to Child and Adolescent Mental Health Services Secondary Uses Data Set
Release: November 2012
- CR1166, CR1167 and CR1306 (1 November 2012) - ISB 0092 Amd-16-2010 Commissioning Data Set Version 6-2, Commissioning Data Set XML Message Version 6-2 and Retirement of CDS 6-0
- CR1305 (1 April 2013) - ISB 0092 Amd 06/2011 Allied Health Professions Referral to Treatment (AHP RTT) Update - CDS 6-2
- CR1286 (1 November 2012) - ISB 0028 Amd 17/2012 Treatment Function Codes Update
- CR1343 (Immediate) - DDCN 1343/2012 Change of name for NHS Commissioning Board Authority
- CR1342 (Immediate) - DDCN 1342/2012 Overseas Visitors Update
- CR1341 (Immediate) - DDCN 1341/2012 Discharge Default Code Descriptions
- CR1323 (Immediate) - National Cancer Waiting Times Monitoring Data Set Update for "Delay Reason To Treatment For Cancer"
CR1323 is a corrigendum to CR1258 (1 July 2012) - ISB 0147 Amd 23/2011 Changes to the National Cancer Waiting Times Monitoring Data Set published in the June 2012 release
The following have been incorporated early to allow users to see the changes, but please note that the implementation date is 1 April 2013:
CR1231 and CR1288 (1 April 2013) - ISB 1570 Amd 164/2010 HIV and AIDS Reporting Data Set and HIV and AIDS Related Data Set Message
Release: September 2012
- CR1103 (Immediate) - ISB 0066 Amd 43/2010 Renal Data Set - Data Item Addition, Changes and Deletions
- CR1334 (Immediate) - DDCN 1334/2012 Psychology Definitions
- CR1331 (Immediate) - DDCN 1331/2012 Activity Date Time Type
- CR1329 (Immediate) - DDCN 1329/2012 Change of name for "Health and Social Care Information Centre"
Release: August 2012
- CR1326 (Immediate) - DDCN 1326/2012 Health and Care Professions Council
- CR1241 (Immediate) - DDCN 1241/2012 NHS dictionary of medicines and devices
- CR1292 (Immediate) - ISB 1549 Amd 4/2011 and DDCN 1292/2012 Deprecation and withdrawal of version 3.2 of the Acute Myocardial Infarction Data Set and subsequent retiring of the Data Set from the NHS Data Model and Dictionary
Release: June 2012
- CR1314 (Immediate) - DDCN 1314/2012 Reasonable Offer Update
- CR1282 (29 June 2012) - ISB 0090 Amd 36/2011 Independent Sector Healthcare Provider (ISHP) Codes extended for ISHPs and Sites
- CR1258 (1 July 2012) - ISB 0147 Amd 23/2011 Changes to the National Cancer Waiting Times Monitoring Data Set
Release: May 2012
- CR1215 (1 June 2012) - ISB 1067 Amd 30/2011 National Workforce Data Set
The following have been incorporated early to allow users to see the changes, but please note that the implementation date is 1 April 2013:
- CR1028 (1 April 2013) - ISB 1069 Amd 14/2012 Children and Young People's Health Services Data Set
- CR1029 (1 April 2013) - ISB 1072 Amd 12/2012 Child and Adolescent Mental Health Services (CAMHS) Data Set
- CR1104 (1 April 2013) - ISB 1513 Amd 13/2012 Maternity Secondary Uses Data Set
Release: March 2012
- CR1242 (Immediate) - DDCN 1242/2012 Retirement of Mental Health Minimum Data Set Version 3
- CR1238 and CR1276 (1 April 2012) - ISB 1577 Amd 10/2011 Diagnostic Imaging Data Set and Diagnostic Imaging Data Set Message v 1-0
- CR1290 (Immediate) - DDCN 1290/2012 Data Set Notation
- CR1263 (Immediate) - ISB 0090 Amd 5/2012 Health and Social Care Bill Changes
- CR1255 (31 March 2012) - ISB 1576 Amd 08/2011 Quarterly Bed Availability and Occupancy Data Set
- CR1295 (Immediate) - Retirement of old Commissioning Data Set messages
The Information Standards Board for Health and Social Care have been involved in the redesign and retirement of the old Commissioning Data Set Pages, however a formal Information Standards Notice (ISN) will not be published as there are no changes to data standards.
Release: January 2012
- CR1285 (Immediate) - DDCN 1285/2012 Elective Admission Type
- CR1252 (Immediate) - DDCN 1252/2011 Geographic Area Changes
Release: November 2011
- CR1264 (Immediate) - ISB 1077 Amd 3/2012 Automatic Identification and Data Capture (AIDC) for Patient Identification Data Set
- CR1274 (Immediate) - DDCN 1274/2011 CDS Prime Recipient Identity Update
The following have been incorporated early to allow users to see the changes, but please note that the implementation date is 1 April 2012:
CR1265 (1 April 2012) - ISB 1520 Amd 29/2011 Changes to the Improving Access to Psychological Therapies Data Set
Release: October 2011
- CR1271 (Immediate) - DDCN 1271/2011 Commissioning Data Set Addressing Grid Update
- CR1268 (Immediate) - DDCN 1268/2011 Sexual Orientation Code
The following has been incorporated early to allow users to see the changes, but please note that the implementation date is 1 April 2012:
- CR1158 and CR1260 (1 April 2012) - ISB 1533 Amd 63/2010 Systemic Anti-Cancer Therapy Data Set and Systemic Anti-Cancer Therapy Data Set Message Schema
The following have been incorporated early to allow users to see the changes, but please note that the implementation date is 1 July 2012:
- CR1270 (1 July 2012) - ISB 1080 Amd 25/2011 Amendments to NHS Health Check Data Set
- CR1250 (1 July 2012) - ISB 1080 Amd 25/2011 NHS Health Checks Data Set Message Schema Version 2.0.0
Release: August 2011
- CR1232 (Immediate) - ISB 0034 Amd 26/2006 Systematized Nomenclature of Medicine Clinical Terms (SNOMED CT) - NHS Data Model and Dictionary Overview
- CR1222 (1 April 2012) - ISB 0021 Amd 86/2010 Introduction of the International Classification of Diseases Tenth Revision 4th Edition
- CR1190 (1 September 2011) - ISB 1538 Amd 131/2010 Chlamydia Testing Activity Data Set
- CR1188 (Immediate) - Amd 85/2010 Genitourinary Medicine Clinic Activity Data Set (GUMCAD) Extension to include Enhanced Sexual Health Services (ESHS)
The following data set is initially being introduced for local use only. A future Information Standards Notice will be published to notify providers and system suppliers of the requirement to flow the data set nationally:
- CR1105 (1 April 2012) - ISB 1510 Amd 25/2010 Community Information Data Set
Release: July 2011
- CR1249 (Immediate) - DDCN 1249/2011 General Pharmaceutical Council Registration Changes
The following has been incorporated early to allow users to see the changes, but please note that the implementation date is 1 July 2012:
- CR1148 (1 July 2012) - ISB 1080 Amd 129/2010 NHS Health Checks Data Set
Release: June 2011
- CR1256 (Immediate) - DDCN 1256/2011 School Definitions
- CR1117 (26 August 2011) - ISB 0090 Amd 94/2010 Organisation Data Service Identification Codes for Local Authorities in England and Wales
- CR1251 (Immediate) - DDCN 1251/2011 Change to the Format/Length of Weekly Hours Worked
- CR1243 (Immediate) - DDCN 1243/2011 National Interim Clinical Imaging Procedure (NICIP) Code Set
Release: April 2011
- CR1154 (1 April 2011) - ISB 0011 Amd 87/2010 Mental Health Minimum Data Set Version 4.0
- CR1234 (Immediate) - DDCN 1234/2011 Technology Reference Data Update Distribution Service (TRUD)
- CR1168 (Immediate) - ISB 0097 Amd 140/2010 Genitourinary Medicine Access Monthly Monitoring Data Set Amendments - Removal of Human Immunodeficiency Virus data
The following has been incorporated early to allow users to see the changes, but please note that the implementation date is 1 April 2012:
- CR1050 (1 April 2012) - ISB 1520 Amd 51/2010 Improving Access to Psychological Therapies Data Set
Release: March 2011
- CR1224 (1 April 2011) - ISB 0092 Amd 02/20110 Commissioning Data Set Schema Version 6-1-1
- CR1223 (Immediate) - DDCN 1223/2011 Updates to Family Planning References
- CR1225 (Immediate) - DDCN 1225/2011 Practitioners with Special Interests
- CR1216 (1 April 2011) - ISB 0028 Amd 170/2010 Changes to Treatment Function Codes
- CR1203 (1 April 2011) - ISB 0084 Amd 150/2010 Introduction of OPCS Classification of Interventions and Procedures Version 4.6
Release: January 2011
- CR1116 (1 April 2010) - ISB 0003 Amd 79/2010 Immunisation Programmes Activity Data Set (KC50)
- CR1112 (1 April 2010) - ISB 1511 Amd 26/2010 NHS Continuing Healthcare and NHS Funded Nursing Care
- CR1068 (Immediate) - ISB 0133 Amd 161/2010 Change To Central Return: Human Papillomavirus (HPV) Immunisation Programme - Vaccine Monitoring Minimum Data Set
- CR1211 (Immediate) - DDCN 1211/2010 Commissioning Data Set Addressing Grid / Organisation Code (Code of Commissioner) Update
Release: December 2010
- CR1175 (1 April 2011) - ISB 1518 Amd 166/2010 Changes to Sexual and Reproductive Health Activity Data Set
- CR1198 (Immediate) - ISB 1067 Amd 165/2010 National Workforce Data Set
- CR1207 (01 December 2010) - ISB 1573 Amd 168/2010 Mixed-Sex Accommodation
- CR1149 (01 January 2011) - ISB 0139 Amd 99/2010 GUMCAD: Change to Genitourinary (GU) Episode Types
Release: November 2010
- CR1119 (Immediate) - DDCN 1119/2010 Organisation Codes Update
- CR1192 (Immediate) - DDCN 1192/2010 Change of name for "Health Solution Wales"
- CR1199 (Immediate) - DDCN 1199/2010 General Pharmaceutical Council and Royal Pharmaceutical Society of Great Britain Update
- CR1189 (Immediate) - DDCN 1189/2010 National Institute for Health and Clinical Excellence
- CR1187 (Immediate) - DDCN 1187/2010 Introduction of the Department for Education
Release: September 2010
- CR1128 (Immediate) - DDCN 1128/2010 Changes to reporting procedures for Overseas Visitors from the European Economic Area and Switzerland
- CR1173 (Immediate) - DDCN 1173/2010 Care Quality Commission Update
- CR1143 (Immediate) - DDCN 1143/2010 General Pharmaceutical Council
- CR1061 (1 October 2010) - ISB 0092/2010 CDS Type 20: Out-patient: Retirement of Default Codes for Out-patient Procedures
- CR1133 (Immediate) - ISB 00289/2010 National Specialty List
Release: August 2010
- The August 2010 Release introduces the NHS Data Model and Dictionary Help Pages.
Release: July 2010
- CR1134 (Immediate - ISB 1067/2010 Amd 109/2010 National Workforce Data Set
- CR1082 (Immediate) - ISB 0153/2010 Critical Care Minimum Data Set
- CR1121 (Immediate) - DSCN 17/2010 Retirement of Data Standard KC60 Central Return
Release: May 2010
- CR957 (Immediate) - DSCN 19/2010 Central Returns: KA34 Ambulance Services
- CR1069 (Immediate) - Redesign of the Commissioning Data Set Pages
The Information Standards Board for Health and Social Care have been involved in the redesign of the Commissioning Data Set Pages and are satisfied that it meets the requirements of the service, however a formal Information Standards Notice (ISN) will not be published as there are no changes to data standards.
Release: March 2010
- CR1123 (1 April 2010) - DSCN 18/2010 Information Standards Notice (ISN)
- CR1139 (Immediate) - DSCN 16/2010 Person Weight
- CR1130 (Immediate) - DSCN 15/2010 Change of name for "The NHS Information Centre for health and social care"
- CR1013 (April 2010) - DSCN 14/2010 Sexual and Reproductive Health Activity Dataset (SRHAD)
- CR1125 (Immediate) - DSCN 13/2010 NHS Data Model and Dictionary Maintenance Update - Policy Definitions
- CR1122 (Immediate) - DSCN 11/2010 Changes to Family Planning References
Release: January 2010
- CR1115 (Immediate) - DSCN 10/2010 Data Standards: Updating of e-Government Interoperability Framework and Government Data Standards Catalogue References
Release: December 2009
- CR1100 (Immediate) - DSCN 25/2009 NHS Prescription Services Update
- CR1045 (1 December 2009) - DSCN 17/2009 Referral to Treatment Clock Stop Administrative Event
- CR1003 (1 December 2009) - DSCN 16/2009 Commissioning Data Sets: Mandation of 18 Week Referral To Treatment Data Items
Release: November 2009
- CR1113 (Immediate) - DSCN 24/2009 Information Standards Board for Health and Social Care Update
- CR1087 (Immediate) - DSCN 23/2009 Health Professions Council Update
- CR1081 (Immediate) - DSCN 22/2009 Data Standards: NHS Data Model and Dictionary Maintenance Update
- CR1019 (27 November 2009) - DSCN 21/2009 Data Standards: Organisation Data Service (ODS) - Optical Sites and Optical Headquarters
- CR1034 (27 November 2009) - DSCN 20/2009 Data Standards: Organisation Data Service (ODS) - Care Homes in England and Wales and their Headquarters
Release: September 2009
- CR1065 (1 October 2009) - DSCN 15/2009 Data Standards: Organisation Data Service, Local Health Boards
Release: June 2009
- CR1014 (1 June 2009) - DSCN 13/2009 Religious and Other Belief System Affiliation
- CR1074 (Immediate) - DSCN 12/2009 Data Standards: Care Quality Commission
- CR1056 (Immediate) - DSCN 11/2009 Data Standards: NHS Data Model and Dictionary Maintenance Update
- CR1072 (1 December 2009) - DSCN 10/2009 Data Standards: National Radiotherapy Data Set
- CR1073 (Immediate) - DSCN 09/2009 Central Returns: Diagnostic Waiting Times and Activity Data Set
- CR1066 (Immediate) - DSCN 08/2009 Data Standards: NHS Prescription Services and NHS Dental Services
- CR1047 (1 April 2009) - DSCN 07/2009 Data Standards: Diabetic Retinopathy Screening Dataset v3.6
- CR1059 (Immediate) - DSCN 06/2009 Data Standard: National Workforce Data Set v2.1
- CR914 (April 2008 (Retrospective)) - DSCN 05/2009 NHS Stop Smoking Services Quarterly Monitoring Return
- CR899 (Immediate) - DSCN 02/2009 NHS Data Model and Dictionary Maintenance Update
Release: March 2009
- CR1001 (1 April 2009) - DSCN 03/2009 Introduction of Commissioning Data Set Schema Version 6-1 (2008-04-01) and update to Commissioning Data Set Schema Version 6-0 (2008-01-14)
- CR976 (31 March 2009) - DSCN 26/2008 Subject: KP90 - Admissions, Changes in Status and Detentions under the Mental Health Act
- CR1017 (1 April 2009) - DSCN 25/2008 Critical Care Minimum Data Set
- CR1002 (1 April 2009) - DSCN 24/2008 Data Standards: Introduction of Commissioning Dataset Version 6.1
- CR1016 (Immediate) - DSCN 23/2008 4 Byte Version of the Read Codes - Withdrawal
Release: December 2008
- CR1022 (1 January 2009) - DSCN 29/2008 Data Standards: 18 Weeks Referral to Treatment (RTT) Time, Performance Sharing
- CR901 (Immediate) - DSCN 28/2008 Removal of references to EDIFACT and the NHS Wide Clearing Service (NWCS)
- CR843 (1 April 2009) - DSCN 22/2008 Data Standards: National Radiotherapy Data Set
- CR1011 (1 January 2009) - DSCN 20/2008 Data Standards: National Cancer Waiting Times Minimum Data Set
Release: November 2008
- CR1026 (3 November 2008) - DSCN 21/2008 Information Standard: Mental Health Act 2007 Mental Category
Release: August 2008
- CR1018 (Immediate) - DSCN 19/2008 Data Standards: Change of Name for National Administrative Code Services (NACS) to Organisation Data Service (ODS)
- CR956 (1 September 2008) - DSCN 18/2008 Central Return: Human Papillomavirus (HPV) Immunisation Programme, Vaccine Monitoring Minimum Dataset
- CR861 (Immediate) - DSCN 16/2008 Central Return: Hospital and Community Services Complaints and General Practice (including Dental) Complaints - KO41(a) and KO 41(b)
- CR964 (Immediate) - DSCN 14/2008 Central Return: 18 Weeks ‘Adjusted’ Referral to Treatment (RTT) Dataset
- CR965 (Immediate) - DSCN 13/2008 Data Standards: Organisation Data Service (ODS) - Change to the Default Codes Set to Support Changes to GMS Contract
- CR879 (Immediate) - DSCN 12/2008 Data Standards: Quarterly Monitoring: Cancelled Operations Data Set (QMCO)
Release: May 2008
- CR502 (Immediate) - DSCN 10/2008 Data Standards: National Workforce Data Definitions (v2.0)
- CR910 (1 April 2008) - DSCN 08/2008 Data Standards: National Direct Access Audiology Patient Tracking List (PTL) and Waiting Times (WT) data sets
- CR900 (Immediate) - DSCN 07/2008 Data Standards: Inter-Provider Transfer Administrative Minimum Data Set
- CR934 (1 April 2008) - DSCN 06/2008 Data Standards: Mental Health Minimum Data Set (version 3.0)
- CR935 (Immediate) - DSCN 05/2008 Data Standards: 18 Weeks Rules Suite
- CR925 (1 September 2008) - DSCN 04/2008 Genitourinary Medicine Clinic Activity Data Set Change to an Information Standard
- CR942 (1 June 2008) - DSCN 03/2008 General Practice and General Medical Practitioner (GMP) - changes resulting from the introduction of the General Medical Services (GMS) Contract
Release: February 2008
- CR812 (Immediate) - DSCN 01/2008 Central Return: Diagnostics Waiting Times Census Data Set
- CR881 (31 December 2007) - DSCN 42/2007 Central Return: Referral To Treatment Summary Patient Tracking List
- CR904 (Immediate) - DSCN 41/2007 Data Standards: Admission Intended Procedure Update
- CR824 (1 February 2008) - DSCN 39/2007 Data Standards: 48 Hour Genitourinary Medicine Access Monthly Monitoring (GUMAMM)
Release: November 2007
- CR919 (Immediate) - DSCN 38/2007 Data Standards: Mental Health Minimum Data Set Schema
- CR814 (1 April 2008) - DSCN 37/2007 Data Standards: Introduction of Mental Health Minimum Data Set version 2.1
- CR930 (31 December 2007) - DSCN 35/2007 Data Standards: A correction to the version 6 Commissioning Data Set schema
- CR834 (Immediate) - DSCN 34/2007 Data Standards: Referral Request Received Date
- CR875 (Immediate) - DSCN 33/2007 Data Standards: National Administrative Codes Service: Introduction of codes for the new Pan SHAs
- CR880 (Immediate) - DSCN 29/2007 Data Standards: Amendments to Doctor Index Number (DIN) Description
Release: August 2007
- CR845 (Immediate) - DSCN 28/2007 Data Standards: Treatment Function Code (Referral to Treatment Period)
- CR831 (1 October 2007) - DSCN 27/2007 Data Standards: Update to Commissioning Data Set XML Schema v5
- CR825 (1 October 2007) - DSCN 16/2007 Data Standards: Source of Referral for Outpatients (18 Weeks)
Release: June 2007
- CR799 (31 December 2007) - DSCN 18/2007 Data Standards: Introduction of Commissioning Data Set Version 6
- CR833 (Immediate) - DSCN 17/2007 Data Standards: Introduction of Commissioning Data Set validation table
- CR801 (Immediate) - DSCN 15/2007 Data Standards: Cover of Vaccination Evaluated Rapidly (COVER) Return
Release: May 2007
- CR800 (31 December 2007) - DSCN 14/2007 Commissioning Data Set Schema Version 6-0
- CR856 (1 October 2007) - DSCN 13/2007 Data Standards: Discharge Ready Date
- CR869 (Immediate) - DSCN 12/2007 Data Standards: Update to Clinical Coding Introduction
- CR827 (1 October 2007) - DSCN 09/2007 Data Standards: Earliest Reasonable Offer Date
- CR817 (1 October 2007) - DSCN 08/2007 Data Standards: Introduction of Age into Commissioning Data Sets
- CR849 (May 2007) - DSCN 07/2007 National Administrative Codes Service: Introduction of new identification codes for Dental Consultants
- CR822 (Immediate) - DSCN 06/2007 Data Standards: Update to Organisation Codes
- CR850 (Immediate) - DSCN 05/2007 National Administrative Codes Service: Amendments to Default Codes
- CR786 (1 April 2007) - DSCN 04/2007 Quarterly Monitoring Accident and Emergency Services (QMAE) Central Return
Release: February 2007
- CR811 (Immediate) - DSCN 03/2007 Diagnostic Waiting Times and Activity
- CR826 (1 October 2007) - DSCN 02/2007 Extension of Treatment Function to Support the Measurement of 18 Week Referral to Treatment Periods
- CR813 (1 April 2007) - DSCN 01/2007 Paediatric Critical Care Minimum Data Set
- CR768 (1 January 2007) - DSCN 18/2006 Changes to the NHS Data Dictionary to support the measurement of 18 week referral to treatment periods
- CR798 (6 November 2006) - DSCN 19/2006 Commissioning Data Set (CDS) Version 5 XML Message Schema
- CR776 (1 October 2006) - DSCN 05/2006 Data Standards: Accident and Emergency Enhancements to Investigation and Treatment Codes
Release: September 2006
- CR795 (31 October 2006) - DSCN 22/2006 Organisation Codes / Organisation Site Codes
- CR792 (1 April 2007) - DSCN 15/2006 Neonatal Critical Care
- CR719 (1 April 2006) - DSCN 09/2006 Measuring and Recording of Waiting Times
- CR791 (1 April 2007) - DSCN 13/2006 Priority Type
- CR774 (1 September 2006) - DSCN 12/2006 Person Marital Status
Release: May 2006
- CR764 (1 April 2006) - DSCN 08/2006 Diagnostics waiting times and activity
- Correction to menu structure to include Critical Care Minimum Data Set
Release: April 2006
- CR608 (1 October 2006) - DSCN 07/2006 Introduction of Commissioning Data Set Version 5 and its associated XML schema into the NHS Data Dictionary.
- CR756 (1 September 2005) - DSCN 19/2005 PbR Commissioning for Out of Area Treatments (OATs) and Charge-Exempt Overseas Visitors
- CR724 (1 April 2006) - DSCN 13/2005 Critical Care Minimum Data Set
- CR754 (1 April 2006) - DSCN 17/2005 Treatment Function and Main Specialty Code Revisions
- CR763 (1 April 2006) - DSCN 20/2005 New Treatment Functions for therapy services and anticoagulant service
- CR767 (Immediate) - DSCN 02/2006 Referral Request Received Date
- CR690 (1 September 2005) - DSCN 16/2005 Marital Status
Release: August 2005
- CR555 (1 April 2005) - DSCN 11/2005 Data Standards: COVER - Hepatitis B immunisation for babies
- CR715 (Immediate) - DSCN 10/2005 Data Standards: Treatment Function Codes - correction and clarification of names and descriptions
- CR706 (1 April 2005) - DSCN 09/2005 Data Standards: Cancer Registration Data Set
- CR691 (1 July 2005) - DSCN 06/2005 Data Standards: NSCAG Commissioner Code
Change to Class: Changed Attributes
| A and E PATIENT GROUP | ||
| ACTIVITY GROUP TYPE | ||
| ADMISSION METHOD | ||
| CANCER OR SYMPTOMATIC BREAST REFERRAL PATIENT STATUS | ||
| CANCER TRANSFER REASON FOR INTER PROVIDER TRANSFER | ||
| CANCER TREATMENT INTENT | ||
| CARER RESIDENT INDICATION CODE FOR NATIONAL NEONATAL DATA SET | ||
| CHILDREN TEENAGERS AND YOUNG ADULTS AGE CATEGORY | ||
| COMMUNITY TREATMENT ORDER END REASON | ||
| CONSULTANT EPISODE COMPLETION STATUS FOR PATIENT LEVEL INFORMATION COSTING | ||
| CONTINUITY OF CARER PATHWAY INDICATOR | ||
| DAUGHTER BORN AT THIS ENCOUNTER INDICATOR | ||
| DELIVERY PLACE CHANGE REASON | ||
| DISCHARGE DESTINATION | ||
| DISCHARGED TO HOSPITAL AT HOME SERVICE INDICATOR | ||
| DISCHARGE FROM IMPROVING ACCESS TO PSYCHOLOGICAL THERAPIES SERVICE REASON | ||
| DISCHARGE METHOD | ||
| DISCHARGE REASON FOR MOTHER MATERNITY SERVICES | ||
| ESTIMATED DATE OF DELIVERY | ||
| FIRST REGULAR DAY OR NIGHT ADMISSION | ||
| FITNESS ASSESSMENT FOR OLDER PATIENTS WITH BREAST CANCER INDICATOR | ||
| HOLISTIC NEEDS ASSESSMENT POINT OF PATHWAY FOR CANCER | ||
| LAST EPISODE IN SPELL INDICATOR CODE | ||
| LENGTH OF STAY ADJUSTMENT | ||
| LENGTH OF STAY ADJUSTMENT REASON | ||
| MATERNAL CRITICAL INCIDENT INDICATOR | ||
| MENTAL HEALTH ABSOLUTE DISCHARGE RESPONSIBILITY | ||
| MENTAL HEALTH ADMITTED PATIENT CLASSIFICATION | ||
| MENTAL HEALTH CONDITIONAL DISCHARGE END REASON | ||
| MENTAL HEALTH DELAYED DISCHARGE ATTRIBUTABLE TO INDICATION CODE | ||
| MENTAL HEALTH DELAYED DISCHARGE REASON | ||
| NEONATAL CRITICAL INCIDENT INDICATOR | ||
| NEONATAL LEVEL OF CARE | ||
| NHS CONTINUING HEALTHCARE COMMISSIONED SERVICES INDICATOR | ||
| NHS CONTINUING HEALTHCARE REFERRAL EXCEEDING 28 DAYS TIME BAND CATEGORY | ||
| NHS CONTINUING HEALTHCARE TYPE | ||
| NON SMOKING CONFIRMATION STATUS AT 4 WEEKS | ||
| OPERATION FUNDING FOR NATIONAL JOINT REGISTRY | ||
| OUTCOME AT 4 WEEK FOLLOW UP FOR STOP SMOKING | ||
| PALLIATIVE CARE SPECIALIST SEEN INDICATOR | ||
| PALLIATIVE TREATMENT REASON FOR UPPER GASTROINTESTINAL | ||
| PATIENT ATTENDANCE SYMPTOMATIC INDICATOR FOR SEXUAL HEALTH SERVICE | ||
| PATIENT CLASSIFICATION | ||
| PATIENT RECEIVING ONE TO ONE NURSING CARE INDICATOR | ||
| PERSONALISED CARE AND SUPPORT PLANNING POINT OF CANCER PATHWAY | ||
| PHARMACOTHERAPY STOP SMOKING AID RECEIVED | ||
| PLANNED DELIVERY SETTING CHANGE REASON | ||
| PREGNANCY OUTCOME | ||
| PREGNANCY OUTCOME CODE | ||
| PSYCHIATRIC PATIENT STATUS | ||
| SOURCE OF ADMISSION |
Change to Class: Changed Attributes
| A and E ATTENDANCE CATEGORY | ||
| A and E INITIAL ASSESSMENT TRIAGE CATEGORY | ||
| A and E STREAM | ||
| ACCIDENT AND EMERGENCY ARRIVAL MODE | ||
| ACCIDENT AND EMERGENCY ATTENDANCE DISPOSAL | ||
| ACUTE ONCOLOGY ASSESSMENT PATIENT PRESENTATION TYPE | ||
| ACUTE ONCOLOGY EPISODE OUTCOME | ||
| CARE CONTACT CANCELLATION REASON | ||
| CARE CONTACT PATIENT THERAPY MODE | ||
| CARE CONTACT SUBJECT | ||
| CARE CONTACT TYPE | ||
| CARE PROGRAMME APPROACH REVIEW ABUSE QUESTION ASKED INDICATOR | ||
| CHILD DIFFICULT TO TEST REASON | ||
| CLINICAL NURSE SPECIALIST INDICATION CODE | ||
| CLINIC ATTENDANCE PURPOSE CODE FOR HIV | ||
| CONSULTATION TYPE | ||
| EMERGENCY CARE ATTENDANCE CATEGORY | ||
| FIRST ATTENDANCE | ||
| GROUP THERAPY INDICATOR | ||
| INFORMATION AND ADVICE PROVIDED INDICATOR | ||
| INFORMATION AND ADVICE TYPE PROVIDED FOR FEMALE GENITAL MUTILATION | ||
| INITIAL CONTACT INDICATOR | ||
| INITIAL DIAGNOSIS CARE SETTING OR SERVICE FOR HIV | ||
| INTERPRETER PRESENT AT CARE CONTACT INDICATION CODE | ||
| LATE ANTENATAL BOOKING APPOINTMENT REASON | ||
| MEDICAL STAFF TYPE SEEING PATIENT | ||
| MENTAL HEALTH PREDICTION AND DETECTION INDICATOR | ||
| MULTIPROFESSIONAL OR MULTIDISCIPLINARY INDICATION CODE | ||
| NEW HIV DIAGNOSIS IN UNITED KINGDOM INDICATOR | ||
| OTHER PERSON IN ATTENDANCE AT CARE CONTACT | ||
| OUTCOME OF ATTENDANCE | ||
| OUT PATIENT ATTENDANCE INDICATOR FOR RADIOTHERAPY DATA SET | ||
| PATIENT HIV CARE STATUS | ||
| POST EXPOSURE PROPHYLAXIS INDICATOR | ||
| PRE EXPOSURE PROPHYLAXIS INDICATOR | ||
| PSYCHIATRIC CARE INDICATOR FOR HIV | ||
| SKIN TO SKIN CONTACT INDICATOR | ||
| STAFF ROLE CARRYING OUT HOLISTIC NEEDS ASSESSMENT OR PERSONALISED CARE AND SUPPORT PLANNING | ||
| TWO YEAR NEONATAL OUTCOMES ASSESSMENT NOT CARRIED OUT REASON |
Change to Class: Changed Attributes
| ABDOMINAL XRAY PERFORMED REASON | ||
| ABDOMINAL XRAY PERFORMED TO INVESTIGATE ABDOMINAL SIGNS INDICATOR | ||
| ABLATIVE THERAPY TYPE | ||
| ACCIDENT AND EMERGENCY INVESTIGATION | ||
| ACCIDENT AND EMERGENCY TREATMENT | ||
| ACUTE ONCOLOGY ASSESSMENT PATIENT PRESENTATION TYPE | ||
| ACUTE ONCOLOGY EPISODE OUTCOME | ||
| ADDITIONAL UNPLANNED PROCEDURE REQUIRED INDICATOR | ||
| ADJUNCTIVE THERAPY TYPE | ||
| ANAESTHETIC TYPE FOR JOINT REPLACEMENT | ||
| ANTIRETROVIRAL THERAPY DRUG REGIMEN GROUP CODE | ||
| ANTIRETROVIRAL THERAPY HOME DELIVERY INDICATOR | ||
| ARTHROPLASTY REVISION TYPE FOR HIP KNEE AND ANKLE REPLACEMENT | ||
| ARTHROPLASTY REVISION TYPE FOR SHOULDER AND ELBOW REPLACEMENT | ||
| ASA PHYSICAL STATUS CLASSIFICATION SYSTEM CODE | ||
| ASA PHYSICAL STATUS CLASSIFICATION SYSTEM CODE FOR NATIONAL JOINT REGISTRY | ||
| ASSOCIATED PROCEDURE TYPE FOR ANKLE REPLACEMENT | ||
| BIOLOGICAL GLENOID RESURFACING TYPE FOR SHOULDER REPLACEMENT | ||
| BIOPSY ANAESTHETIC TYPE | ||
| BIOPSY TYPE FOR CENTRAL NERVOUS SYSTEM TUMOURS | ||
| BLOOD PRODUCTS REQUIRED FOLLOWING OESOPHAGECTOMY INDICATION CODE | ||
| BLOOD TRANSFUSION PRODUCT TYPE | ||
| BLOOD TRANSFUSION TYPE | ||
| BONE GRAFT INDICATOR FOR JOINT REPLACEMENT | ||
| BONE GRAFT SOURCE FOR JOINT REPLACEMENT | ||
| BONE GRAFT STRUCTURE FOR JOINT REPLACEMENT | ||
| BREAST ASSESSMENT OUTCOME | ||
| BREAST TRIPLE DIAGNOSTIC ASSESSMENT INDICATOR | ||
| BRONCHOSCOPY PERFORMED TYPE | ||
| CANCER CARE SETTING FOR TREATMENT | ||
| CANCER IMAGING MODALITY | ||
| CANCER IMAGING OUTCOME | ||
| CANCER SURGICAL ADMISSION TYPE | ||
| CANCER TREATMENT MODALITY | ||
| CARDIOPULMONARY EXERCISE TEST TYPE | ||
| CEMENT REMOVAL INDICATOR FOR JOINT REPLACEMENT | ||
| CHEMICAL THROMBOPROPHYLAXIS REGIME TYPE FOR JOINT REPLACEMENT | ||
| CHEST DRAIN IN SITU INDICATOR | ||
| CLINICAL INTERVENTION TEXT STRING | ||
| CLINICAL INTERVENTION TYPE | ||
| CO MORBIDITY ADJUSTMENT INDICATOR | ||
| COMPONENT REMOVAL INDICATOR FOR JOINT REPLACEMENT | ||
| COMPUTER GUIDED SURGERY INDICATOR FOR JOINT REPLACEMENT | ||
| CONTINUOUS INFUSION OF PULMONARY VASODILATOR RECEIVED INDICATOR | ||
| CONTRACEPTION METHOD STATUS | ||
| DEINFIBULATION UNDERTAKEN REASON | ||
| DELIVERED IN WATER INDICATOR | ||
| DELIVERY INSTRUMENT TYPE | ||
| DIEPOXYBUTANE TEST RESULT | ||
| DRUG REGIMEN ACRONYM | ||
| ENDOSCOPIC OR RADIOLOGICAL COMPLICATION TYPE | ||
| ENDOSCOPIC PROCEDURE TYPE | ||
| ENTERAL FEEDING METHOD | ||
| ENTERAL FEED TYPE GIVEN | ||
| ESCALATION IN LEVEL OF PATIENT CARE FOLLOWING OESOPHAGECTOMY INDICATOR | ||
| EXCISION TYPE FOR CENTRAL NERVOUS SYSTEM TUMOURS | ||
| FETAL ORDER | ||
| FIRST ANTIRETROVIRAL THERAPY IN THE UNITED KINGDOM INDICATOR | ||
| FIXATION TYPE FOR ELBOW REPLACEMENT | ||
| FIXATION TYPE FOR SHOULDER REPLACEMENT | ||
| FORMULA MILK OR MILK FORTIFIER TYPE | ||
| FRACTION NUMBER | ||
| GERMLINE GENETIC TEST TYPE OFFERED | ||
| HIP JOINT SURGERY PATIENT POSITION | ||
| HUMAN PAPILLOMAVIRUS VACCINATION DOSE GIVEN | ||
| IMAGE GUIDED SURGERY INDICATOR | ||
| IMAGING ANATOMICAL SITE | ||
| INFECTION CULTURE TEST INDICATOR | ||
| INTERNATIONAL ESOPHAGEAL DATABASE SURGICAL COMPLICATIONS | ||
| INTERVENTION SESSION TYPE FOR STOP SMOKING | ||
| INTERVENTION SETTING TYPE FOR STOP SMOKING | ||
| INTRAPARTUM ANTIBIOTICS GIVEN INDICATOR | ||
| INTRAVESICAL CHEMOTHERAPY RECEIVED INDICATOR | ||
| INTRAVESICAL IMMUNOTHERAPY RECEIVED INDICATOR | ||
| INVESTIGATION SCHEME IN USE | ||
| JOINT REPLACEMENT PATIENT PROCEDURE PERFORMED INDICATOR | ||
| JOINT REPLACEMENT REVISION REASON CODE FOR ANKLE | ||
| JOINT REPLACEMENT REVISION REASON CODE FOR ELBOW | ||
| JOINT REPLACEMENT REVISION REASON CODE FOR HIP | ||
| JOINT REPLACEMENT REVISION REASON CODE FOR KNEE | ||
| JOINT REPLACEMENT REVISION REASON CODE FOR SHOULDER | ||
| KI 67 STAINING PERFORMED INDICATION CODE | ||
| LABOUR OR DELIVERY ONSET METHOD | ||
| LABOUR OR DELIVERY ONSET METHOD CODE FOR NATIONAL NEONATAL DATA SET | ||
| LAPAROTOMY FOR NECROTISING ENTEROCOLITIS INDICATION CODE | ||
| LINER REMOVAL INDICATOR FOR JOINT REPLACEMENT | ||
| LIVER CANCER SURVEILLANCE SCAN INDICATOR | ||
| LIVER SURGERY PERFORMED TYPE | ||
| LIVER TRANSARTERIAL EMBOLISATION MATERIAL INJECTION TYPE | ||
| MARGIN INVOLVED INDICATION CODE | ||
| MARGIN INVOLVED INDICATION CODE FOR COLORECTAL | ||
| MATERNITY CARE SETTING | ||
| MECHANICAL THROMBOPROPHYLAXIS REGIME TYPE FOR JOINT REPLACEMENT | ||
| MECONIUM PRESENT IN LIQUOR INDICATOR | ||
| MINIMALLY INVASIVE OESOPHAGECTOMY SURGICAL APPROACH TYPE | ||
| MINIMALLY INVASIVE SURGERY INDICATOR FOR JOINT REPLACEMENT | ||
| MORE THAN THREE RECTAL WASHOUTS RECEIVED INDICATOR | ||
| NEOADJUVANT THERAPY INDICATOR | ||
| NEONATAL RESUSCITATION METHOD FOR NATIONAL NEONATAL DATA SET | ||
| NEURODEVELOPMENTAL ASSESSMENT ALREADY TAKEN INDICATOR | ||
| NEWBORN HEARING SCREENING TEST TYPE | ||
| NITRIC OXIDE GIVEN INDICATOR | ||
| NUMBER OF TELETHERAPY FIELDS | ||
| OBSERVATION SCHEME IN USE | ||
| OESOPHAGECTOMY ANASTOMOSIS TYPE | ||
| OESOPHAGECTOMY NECK DISSECTION INDICATOR | ||
| OESOPHAGECTOMY OESOPHAGEAL CONDUIT TYPE | ||
| OESOPHAGECTOMY SURGICAL APPROACH TYPE | ||
| OPEN OESOPHAGECTOMY SURGICAL APPROACH TYPE | ||
| OPERATION STATUS CODE | ||
| PARENTAL CONSENT TO ADMINISTER VITAMIN K INDICATOR | ||
| PARENTAL CONSENT TO POST MORTEM INDICATOR | ||
| PARENTERAL NUTRITION RECEIVED INDICATOR | ||
| PATHOLOGY INVESTIGATION TYPE | ||
| PATHOLOGY INVESTIGATION TYPE FOR BREAST SCREENING | ||
| PATIENT CONSENT FOR TISSUE BANKED AT DIAGNOSIS INDICATION CODE | ||
| PATIENT DIAGNOSIS TREATMENT PROVIDED INDICATION CODE FOR SEXUAL HEALTH SERVICE | ||
| PATIENT PROCEDURE PERFORMED INDICATOR | ||
| PATIENT PROCEDURE TYPE FOR PRIMARY ANKLE REPLACEMENT | ||
| PATIENT PROCEDURE TYPE FOR PRIMARY ELBOW REPLACEMENT | ||
| PATIENT PROCEDURE TYPE FOR PRIMARY HIP REPLACEMENT | ||
| PATIENT PROCEDURE TYPE FOR PRIMARY KNEE REPLACEMENT | ||
| PATIENT PROCEDURE TYPE FOR PRIMARY SHOULDER REPLACEMENT | ||
| PATIENT PROCEDURE TYPE FOR REVISION ANKLE REPLACEMENT | ||
| PATIENT PROCEDURE TYPE FOR REVISION ELBOW REPLACEMENT | ||
| PATIENT PROCEDURE TYPE FOR REVISION HIP REPLACEMENT | ||
| PATIENT PROCEDURE TYPE FOR REVISION KNEE REPLACEMENT | ||
| PATIENT PROCEDURE TYPE FOR REVISION SHOULDER REPLACEMENT | ||
| PATIENT SPECIFIC INSTRUMENTS INDICATOR FOR SHOULDER OR KNEE REPLACEMENT | ||
| PATIENT TREATED TO CHILDRENS CANCER AND LEUKAEMIA GROUP GUIDELINES INDICATOR | ||
| PLANE OF SURGICAL EXCISION INDICATOR | ||
| POST MORTEM CARRIED OUT INDICATOR | ||
| POST MORTEM CONFIRMED NECROTISING ENTEROCOLITIS DIAGNOSIS INDICATOR | ||
| PRETREATMENT PROSTATE BIOPSY TECHNIQUE TYPE | ||
| PREVIOUS BONY INFECTION INDICATOR OF TIBIA OR HINDFOOT FOR ANKLE REPLACEMENT | ||
| PREVIOUS FRACTURE OF INDEX JOINT INDICATOR FOR ANKLE REPLACEMENT | ||
| PREVIOUS INDEX JOINT SURGERY TYPE FOR ANKLE REPLACEMENT | ||
| PREVIOUS SURGERY TYPE FOR SHOULDER REPLACEMENT | ||
| PRIMARY INDUCTION CHEMOTHERAPY FAILURE INDICATOR | ||
| PRINCIPAL DIAGNOSTIC IMAGING TYPE | ||
| PROCEDURE SCHEME IN USE | ||
| PROSTATE NERVE SPARING SURGERY TYPE | ||
| RADICAL PROSTATECTOMY MARGIN STATUS | ||
| RADIOISOTOPE | ||
| RADIOTHERAPY ACTUAL DOSE | ||
| RADIOTHERAPY BEAM TYPE | ||
| RADIOTHERAPY INTENT | ||
| RADIOTHERAPY PRESCRIBED DOSE | ||
| RADIOTHERAPY TREATMENT MODALITY | ||
| REGIONAL ANAESTHETIC TECHNIQUE FOR CANCER | ||
| RELAPSE METHOD DETECTION TYPE | ||
| RENAL VEIN TUMOUR INDICATOR FOR PAEDIATRIC KIDNEY | ||
| RENAL VEIN TUMOUR THROMBUS INDICATION CODE FOR UROLOGICAL | ||
| REPLOGLE TUBE IN SITU INDICATOR | ||
| RESPIRATORY SUPPORT DEVICE TYPE FOR NATIONAL NEONATAL DATA SET | ||
| RESPIRATORY SUPPORT MODE FOR NATIONAL NEONATAL DATA SET | ||
| RESUSCITATION METHOD CODE | ||
| RETINOPATHY OF PREMATURITY SCREENING OUTCOME STATUS CODE | ||
| REVISION PROCEDURE TYPE FOR ANKLE REPLACEMENT | ||
| REVISION PROCEDURE TYPE FOR ELBOW REPLACEMENT | ||
| REVISION PROCEDURE TYPE FOR HIP REPLACEMENT | ||
| REVISION PROCEDURE TYPE FOR KNEE REPLACEMENT | ||
| REVISION PROCEDURE TYPE FOR SHOULDER REPLACEMENT | ||
| ROTATOR CUFF CONDITION FOR SHOULDER REPLACEMENT | ||
| ROTATOR CUFF REPAIRED INDICATOR FOR SHOULDER REPLACEMENT | ||
| ROTATOR CUFF REPAIR TYPE FOR SHOULDER REPLACEMENT | ||
| SENTINEL LYMPH NODE BIOPSY TYPE | ||
| SIGNIFICANT MATERNAL PYREXIA IN LABOUR INDICATOR | ||
| STAFF ROLE CARRYING OUT HOLISTIC NEEDS ASSESSMENT OR PERSONALISED CARE AND SUPPORT PLANNING | ||
| STEM CELL INFUSION DONOR TYPE | ||
| STEM CELL INFUSION SOURCE CODE | ||
| STEM CELL TRANSPLANT CONDITIONING REGIMEN | ||
| STEROIDS GIVEN DURING PREGNANCY TO MATURE FETAL LUNGS INDICATOR | ||
| STOMA PRESENT INDICATOR | ||
| SURFACTANT GIVEN INDICATOR | ||
| SURGICAL ACCESS TYPE | ||
| SURGICAL ACCESS TYPE FOR HEAD AND NECK CANCER | ||
| SURGICAL APPROACH FOR PRIMARY HIP REPLACEMENT | ||
| SURGICAL APPROACH FOR PRIMARY KNEE REPLACEMENT | ||
| SURGICAL APPROACH FOR PRIMARY OR REVISION ANKLE REPLACEMENT | ||
| SURGICAL APPROACH FOR PRIMARY OR REVISION ELBOW REPLACEMENT | ||
| SURGICAL APPROACH FOR PRIMARY OR REVISION SHOULDER REPLACEMENT | ||
| SURGICAL APPROACH FOR REVISION HIP REPLACEMENT | ||
| SURGICAL APPROACH FOR REVISION KNEE REPLACEMENT | ||
| SURGICAL PALLIATION TYPE | ||
| SYSTEMIC ANTI CANCER THERAPY CURATIVE TREATMENT COMPLETED AS PLANNED INDICATOR | ||
| SYSTEMIC ANTI CANCER THERAPY CURATIVE TREATMENT NOT COMPLETED OUTCOME REASON | ||
| SYSTEMIC ANTI CANCER THERAPY DRUG REGIMEN MODIFICATION INDICATOR FOR DOSE REDUCTION | ||
| SYSTEMIC ANTI CANCER THERAPY DRUG REGIMEN TREATMENT INTENT | ||
| SYSTEMIC ANTI CANCER THERAPY DRUG ROUTE OF ADMINISTRATION | ||
| SYSTEMIC ANTI CANCER THERAPY NON CURATIVE TREATMENT PATIENT BENEFIT INDICATOR | ||
| TRACHEOSTOMY TUBE IN SITU INDICATOR | ||
| TREATMENT TYPE FOR NECROTISING ENTEROCOLITIS | ||
| TREATMENT TYPE FOR PATENT DUCTUS ARTERIOSUS | ||
| UNITS OF BLOOD TRANSFUSED FOLLOWING OESOPHAGECTOMY | ||
| UNTOWARD INTRAOPERATIVE EVENT CODE FOR ANKLE REPLACEMENT | ||
| UNTOWARD INTRAOPERATIVE EVENT CODE FOR ELBOW REPLACEMENT | ||
| UNTOWARD INTRAOPERATIVE EVENT CODE FOR HIP REPLACEMENT | ||
| UNTOWARD INTRAOPERATIVE EVENT CODE FOR KNEE REPLACEMENT | ||
| UNTOWARD INTRAOPERATIVE EVENT CODE FOR SHOULDER REPLACEMENT | ||
| VASCULAR LINE TYPE IN SITU | ||
| VISUAL INSPECTION CONFIRMED NECROTISING ENTEROCOLITIS DURING LAPAROTOMY INDICATOR | ||
| VITAMIN K ADMINISTERED INDICATOR | ||
| VITAMIN K ROUTE OF ADMINISTRATION |
Change to Class: Changed Attributes
| K | INVESTIGATION RESULT DATE | |
| ABNORMALITY DETECTED INDICATOR | ||
| ACUTE MYELOID LEUKAEMIA RISK FACTORS | ||
| ALK GENE FUSION STATUS | ||
| ANKLE DORSIFLEXION CODE FOR PRIMARY ANKLE REPLACEMENT | ||
| ANKLE PLANTARFLEXION CODE FOR PRIMARY ANKLE REPLACEMENT | ||
| BLOOD PRODUCTS REQUIRED FOLLOWING OESOPHAGECTOMY INDICATION CODE | ||
| BREAST BIOPSY REFERRAL OUTCOME | ||
| BREAST CANCER HISTOLOGICAL TYPE | ||
| BREAST PROGESTERONE RECEPTOR STATUS | ||
| BREAST SCREENING MAMMOGRAPHY OUTCOME CODE | ||
| BRONCHOSCOPY PERFORMED TYPE | ||
| CANCER SPECIMEN NATURE | ||
| CANCER SURGICAL ADMISSION TYPE | ||
| CANCER VASCULAR OR LYMPHATIC INVASION | ||
| CENTRAL TONE STATUS | ||
| CERVICAL GLANDULAR INTRAEPITHELIAL NEOPLASIA PRESENCE AND GRADE | ||
| CHLAMYDIA TEST RESULT | ||
| CLINICAL FRAILTY SCALE POINT | ||
| CLINICAL INVESTIGATION ITEM TYPE | ||
| CLINICAL INVESTIGATION ITEM UNIT OF MEASURE | ||
| CLINICAL INVESTIGATION RESULT ANALYSED DATE | ||
| CLINICAL INVESTIGATION RESULT RECEIVED DATE | ||
| CLINICAL INVESTIGATION RESULT VALUE | ||
| CONDITION SEEN IN ABDOMEN DURING XRAY | ||
| CYSTIC PERIVENTRICULAR LEUKOMALACIA OBSERVED DURING CRANIAL ULTRASOUND SCAN INDICATOR | ||
| CYTOGENETIC ABNORMALITY RISK GROUP | ||
| CYTOGENETIC ANALYSIS CODE | ||
| CYTOGENETIC PRESENCE TYPE FOR RHABDOMYOSARCOMA | ||
| CYTOGENETIC RISK GROUP FOR PAEDIATRIC MOLECULAR GENETIC ABNORMALITIES | ||
| DEGREES OF FIXED FLEXION DEFORMITY FOR PRIMARY KNEE REPLACEMENT | ||
| DEGREES OF FLEXION RANGE FOR PRIMARY KNEE REPLACEMENT | ||
| DETRUSOR MUSCLE PRESENCE INDICATION CODE | ||
| EPIDERMAL GROWTH FACTOR RECEPTOR MUTATIONAL STATUS | ||
| ESTROGEN RECEPTOR STATUS | ||
| EUROPEAN LEUKAEMIA NET GENETIC RISK CODE | ||
| EXCISION MARGIN INDICATION CODE | ||
| FINDING SCHEME IN USE | ||
| GENETIC CONFIRMATION INDICATOR | ||
| GRADE OF DIFFERENTIATION | ||
| GYNAECOLOGICIAL CANCER SITE OF PERITONEAL INVOLVEMENT | ||
| HAEMOGLOBINOPATHY INVESTIGATION RESULT CODE FOR NATIONAL NEONATAL DATA SET | ||
| HEPATITIS B INFECTION INDICATION CODE | ||
| HEPATITIS C INFECTION INDICATION CODE | ||
| HORMONE EXPRESSION TYPE | ||
| HUMAN EPIDERMAL GROWTH FACTOR IN SITU HYBRIDISATION RECEPTOR STATUS FOR BREAST | ||
| HUMAN EPIDERMAL GROWTH FACTOR RECEPTOR STATUS FOR BREAST | ||
| HUMAN PAPILLOMAVIRUS IN SITU HYBRIDISATION TEST RESULT | ||
| IMMUNOHISTOCHEMISTRY NUCLEAR EXPRESSION INTACT INDICATION CODE | ||
| INTRAVENTRICULAR HAEMORRHAGE GRADE | ||
| INVASIVE CANCER SPECIAL TYPE INDICATOR | ||
| INVESTIGATION EXAMINATION RESULT | ||
| INVESTIGATION RUBELLA RESULT INDICATOR | ||
| LEUKAEMIC CELLS PRESENT POST MINIMAL RESIDUAL DISEASE INDUCTION PERCENTAGE | ||
| LYMPH NODE STATUS | ||
| METASTASIS EXTENT CODE | ||
| MICROSATELLITE INSTABILITY TESTING RESULT | ||
| MICROSCOPIC INVOLVEMENT INDICATION CODE FOR FALLOPIAN TUBE OR OVARIAN CANCER | ||
| MICROSCOPIC INVOLVEMENT INDICATION CODE FOR UTERINE SEROSA | ||
| MICROSCOPIC INVOLVEMENT INDICATOR FOR PARAMETRIUM OR CERVICAL STROMA | ||
| MICROSCOPIC INVOLVEMENT INDICATOR FOR VAGINAL | ||
| NEWBORN BLOOD SPOT TEST OUTCOME STATUS | ||
| NEWBORN HEARING AUDIOLOGY OUTCOME | ||
| NEWBORN HEARING SCREENING OUTCOME | ||
| NEWBORN HEARING SCREENING OUTCOME FOR NATIONAL NEONATAL DATA SET | ||
| NUMBER OF FETUSES | ||
| OBSERVATION VALUE | ||
| OESOPHAGECTOMY OESOPHAGEAL CONDUIT NECROSIS FAILURE TYPE | ||
| OESOPHAGOENTERIC LEAK SEVERITY TYPE | ||
| OTHER NON BREAST LOCALLY ADVANCED METASTATIC MALIGNANCY INDICATOR | ||
| P16 IMMUNOHISTOCHEMISTRY TEST RESULT | ||
| PAEDIATRIC MYELODYSPLASIA CLINICAL FINDINGS | ||
| PATHOLOGICAL RISK CLASSIFICATION CODE AFTER NEPHRECTOMY | ||
| PATHOLOGICAL RISK CLASSIFICATION CODE AFTER PREOPERATIVE CHEMOTHERAPY | ||
| PD L1 EXPRESSION PERCENTAGE | ||
| PERINEURAL INVASION INDICATOR FOR SKIN | ||
| PERINEURAL INVASION INDICATOR FOR UROLOGICAL | ||
| PERITONEAL INVOLVEMENT INDICATION CODE | ||
| PERSON BLOOD GROUP | ||
| PERSON RHESUS FACTOR | ||
| PORENCEPHALIC CYST VISIBLE DURING CRANIAL ULTRASOUND SCAN INDICATOR | ||
| PREOPERATIVE THERAPY RESPONSE TYPE | ||
| RECURRENT LARYNGEAL NERVE INJURY INVOLVEMENT TYPE | ||
| RENAL VEIN TUMOUR INDICATOR FOR PAEDIATRIC KIDNEY | ||
| RENAL VEIN TUMOUR THROMBUS INDICATION CODE FOR UROLOGICAL | ||
| RETINOPATHY OF PREMATURITY CLOCK HOURS MAXIMUM STAGE | ||
| RETINOPATHY OF PREMATURITY MAXIMUM ZONE | ||
| RETINOPATHY OF PREMATURITY PLUS DISEASE STATUS | ||
| RETINOPATHY OF PREMATURITY STAGE | ||
| ROS1 FUSION STATUS | ||
| S CATEGORY CODE | ||
| SENTINEL LYMPH NODE BIOPSY OUTCOME | ||
| SITUATION SCHEME IN USE | ||
| SPLENOMEGALY INDICATOR | ||
| SUBTALAR JOINT MOVEMENT CODE FOR PRIMARY ANKLE REPLACEMENT | ||
| TIBIA HINDFOOT ALIGNMENT CODE FOR PRIMARY ANKLE REPLACEMENT | ||
| TUMOUR NECROSIS | ||
| TUMOUR NECROSIS INDICATION CODE | ||
| VENTRICULAR DILATION DIAGNOSED DURING CRANIAL ULTRASOUND SCAN INDICATOR | ||
| VIABLE TUMOUR EVIDENCE AT RESECTION MARGIN | ||
| VISUAL ACUITY OR FIELD TEST RESULT |
Change to Class: Changed Attributes
| ANAPLASTIC NEPHROBLASTOMA TYPE | ||
| BONE INVASION INDICATION CODE | ||
| CANCER METASTATIC DISEASE TYPE | ||
| CARTILAGE INVASION INDICATION CODE | ||
| CHYLE LEAK SEVERITY TYPE | ||
| CLARKS LEVEL IV INDICATION CODE | ||
| CYTOLOGY RESULT CODE | ||
| DYSPLASTIC HAEMOPOIESIS TYPE | ||
| EXTENT OF ATELECTASIS | ||
| EXTENT OF METASTATIC SPREAD | ||
| EXTENT OF PLEURAL INVASION | ||
| EXTRACAPSULAR SPREAD INDICATION CODE | ||
| EXTRAMEDULLARY DISEASE SITE | ||
| EXTRANODAL SPREAD INDICATOR | ||
| GENE OR STRATIFICATION BIOMARKER TYPE ANALYSED | ||
| GYNAECOLOGICIAL CAPSULE STATUS | ||
| INTERNATIONAL SOCIETY OF PAEDIATRIC ONCOLOGY TUMOUR LOCAL STAGE | ||
| LARGEST METASTASIS | ||
| LESION GREATER THAN 20MM INDICATION CODE | ||
| LESION SIZE | ||
| LESION VERTICAL THICKNESS GREATER THAN 2MM INDICATION CODE | ||
| LUNG METASTASES SUB STAGE GROUPING | ||
| MACROSCOPIC EXTRAGLANDULAR EXTENSION INDICATION CODE | ||
| MALIGNANT PLEURAL EFFUSION INDICATOR | ||
| MAXIMUM DEPTH OF INVASION | ||
| METASTATIC SITE | ||
| METASTATIC STATUS | ||
| MICROSATELLITE OR IN TRANSIT METASTASIS INDICATION CODE | ||
| MIXED PHENOTYPE ACUTE LEUKAEMIA SYMPTOMS | ||
| MOLECULAR DIAGNOSTIC CODE | ||
| MULTIFOCAL OR SYNCHRONOUS TUMOUR INDICATOR | ||
| MULTIFOCAL TUMOUR INDICATOR FOR BREAST | ||
| MYOMETRIAL INVASION IDENTIFICATION CODE | ||
| NEEDLE CORE BIOPSY RESULT CODE FOR AXILLARY LYMPH NODE | ||
| NEEDLE CORE BIOPSY RESULT CODE FOR BREAST | ||
| NODAL STATUS | ||
| NUMBER OF ABNORMAL NODAL AREAS | ||
| NUMBER OF EXTRANODAL SITES CODE | ||
| OMENTUM INVOLVEMENT INDICATION CODE | ||
| OVARY SURFACE INVOLVEMENT INDICATOR | ||
| PARACERVICAL OR PARAMETRIAL INVOLVEMENT INDICATOR | ||
| PERITONEAL CYTOLOGY RESULT CODE | ||
| PERITONEAL INVOLVEMENT INDICATOR FOR ENDOMETRIAL CANCER | ||
| PERITONEAL WASHINGS IDENTIFIED | ||
| PORTAL VEIN INVASION INDICATION CODE | ||
| POST OPERATIVE TUMOUR SITE FOR UPPER GASTROINTESTINAL | ||
| PRIMARY TUMOUR STATUS | ||
| RESECTION MARGIN INVOLVEMENT INDICATOR | ||
| RESECTION STATUS | ||
| RESIDUAL DISEASE SIZE FOR GYNAECOLOGICAL CANCER | ||
| RETINOBLASTOMA ASSESSMENT LATERALITY | ||
| RHABDOMYOSARCOMA SITE PROGNOSIS CODE | ||
| SARCOMA TUMOUR BREACH IDENTIFIER | ||
| SARCOMA TUMOUR DEPTH | ||
| SARCOMA TUMOUR SUBSITE FOR BONE | ||
| SARCOMA TUMOUR SUBSITE FOR SOFT TISSUE | ||
| SATELLITE TUMOUR NODULES LOCATION | ||
| SKIN CANCER LESION SPECIMEN IDENTIFIER | ||
| SKIN ULCERATION INDICATION CODE | ||
| SMILE INDICATION CODE | ||
| SYNCHRONOUS TUMOUR COLON LOCATION | ||
| SYNCHRONOUS TUMOUR INDICATOR | ||
| TUMOUR GRADE FOR UROLOGY | ||
| TUMOUR INFILTRATING LYMPHOCYTE TYPE | ||
| TUMOUR INVASION INDICATION CODE FOR DIRECT ADRENAL | ||
| TUMOUR INVASION INDICATOR FOR CHILDREN TEENAGERS AND YOUNG ADULTS | ||
| TUMOUR INVASION INDICATOR FOR KIDNEY | ||
| TUMOUR INVASION INDICATOR FOR LUNG | ||
| TUMOUR INVASION INDICATOR FOR PENIS | ||
| TUMOUR INVASION INDICATOR FOR TESTICULAR | ||
| TUMOUR NECROSIS PERCENTAGE | ||
| TUMOUR OR LESION LATERALITY | ||
| TUMOUR OR LESION LOCATION | ||
| TUMOUR REGRESSION INDICATION CODE FOR SKIN | ||
| TUMOUR RUPTURE INDICATOR | ||
| TUMOUR SIZE | ||
| TUMOUR VOLUME AT DIAGNOSIS CODE | ||
| UNDERLYING DISEASE ASSOCIATED WITH MYELODYSPLASIA |
Change to Class: Changed Attributes
| K | PATIENT PATHWAY IDENTIFIER | |
| CANCER FASTER DIAGNOSIS PATHWAY END REASON | ||
| CANCER FASTER DIAGNOSIS PATHWAY EXCLUSION REASON | ||
| CANCER TREATMENT EVENT TYPE | ||
| WAITING TIME MEASUREMENT TYPE |
Change to Class: Changed Attributes
| K | PERSON IDENTIFIER | |
| EX BRITISH ARMED FORCES INDICATOR | ||
| LOOKED AFTER CHILD INDICATOR | ||
| NATIONAL INSURANCE NUMBER | ||
| NEW SEX PARTNERS IN THE LAST THREE MONTHS INDICATOR | ||
| NUMBER OF DAUGHTERS UNDER 18 | ||
| NUMBER OF SEX PARTNERS IN LAST THREE MONTHS CODE | ||
| PARENTAL RESPONSIBILITIES INDICATOR | ||
| PERSON AGE | ||
| PERSON BIRTH DATE | ||
| PERSON BIRTH TIME | ||
| PERSON COUNT | ||
| PRISONER INDICATOR | ||
| SEX WORKER INDICATOR | ||
| YEAR OF BIRTH |
Change to Class: Changed Attributes
| K | POSITION VACANCY IDENTIFIER | |
| POSITION VACANCY END DATE | ||
| POSITION VACANCY FULL TIME EQUIVALENT | ||
| POSITION VACANCY START DATE | ||
| POSITION VACANCY STATUS |
Change to Class: Changed Attributes
| SOCIO ECONOMIC CLASSIFICATION CODE FOR STOP SMOKING |
Change to Attribute: Changed Description
The type of setting in which a CLINICAL INTERVENTION was delivered for a Stop Smoking Service.
National Codes:
| 01 | Service venue (Retired October 2017) |
| 02 | Pharmacy setting (Retired October 2017) |
| 03 | Prison setting (Retired October 2017) |
| 04 | Primary care setting (Retired October 2017) |
| 05 | Hospital ward setting (Retired October 2017) |
| 06 | Dental Practice setting (Retired October 2017) |
| 07 | Military base setting (Retired October 2017) |
| 10 | Community setting |
| 11 | Community Psychiatric setting |
| 12 | Psychiatric Hospital setting |
| 13 | Pharmacy setting |
| 14 | General Dental Practitioner Practice setting |
| 15 | General Medical Practitioner Practice setting |
| 16 | Maternity setting |
| 17 | Children's Centre setting |
| 18 | School setting |
| 19 | Prison setting |
| 20 | Military Base setting |
| 21 | Workplace setting |
| 22 | Hospital setting |
| 96 | Other (Retired October 2017) |
| 98 | Other (not listed) |
Change to Attribute: Changed Description
An indication of whether there is evidence of coagulative Tumour necrosis and if so the type during an Urological Cancer Care Spell.
National Codes:
| Y | Yes - there is evidence of coagulative Tumour necrosis (Retired 1 April 2020) |
| 1 | Macroscopic (confluent) |
| 2 | Microscopic (coagulative) |
| 3 | Not identified (there is no evidence of coagulative Tumour necrosis) |
Change to Attribute: Changed Description
TUMOUR OR LESION LOCATION is the:
- radiologically determined anatomical location of the Lesion(s) or
- surgically determined anatomical location of the Tumour.
National Codes:
| 01 | Frontal lobe |
| 02 | Temporal lobe |
| 03 | Parietal lobe |
| 04 | Occipital lobe |
| 05 | Pineal region |
| 06 | Hypothalamic |
| 07 | Basal ganglia/thalamic |
| 08 | Cerebellar |
| 09 | Midbrain |
| 10 | Pons |
| 11 | Medulla |
| 12 | Fourth ventricle |
| 13 | Third ventricle |
| 14 | Lateral ventricle |
| 15 | Parasagittal/parafalcine dura |
| 16 | Posterior fossa convexity dura |
| 17 | Convexity dura |
| 18 | Petrous temporal bone |
| 19 | Orbital roof |
| 20 | Skull vault |
| 21 | Scalp |
| 22 | Anterior cranial fossa |
| 23 | Middle cranial fossa |
| 24 | Orbital roof (Retired 1 April 2020) |
| 25 | Infratemporal fossa |
| 26 | Pterygopalatine fossa |
| 27 | Anterior clinoid dura |
| 28 | Sphenoid wing dura |
| 29 | Subfrontal dura |
| 30 | Suprasellar dura |
| 31 | Clival dura |
| 32 | Cavernous sinus |
| 33 | Cerebellopontine angle |
| 34 | Jugular bulb |
| 35 | Venous angle dura |
| 36 | Foramen magnum |
| 37 | Cervical intramedullary |
| 38 | Cervical intradural |
| 39 | Cervical extradural |
| 40 | Cervical bony |
| 41 | Thoracic intramedullary |
| 42 | Thoracic intradural |
| 43 | Thoracic extradural |
| 44 | Thoracic bony |
| 45 | Lumbar intramedullary |
| 46 | Lumbar intradural |
| 47 | Lumbar extradural |
| 48 | Lumbar bony |
| 98 | Other (not listed) |
Change to Attribute: Changed Description
The unit of measurement.
National Codes:
| 01 | Millimoles per litre (mmol/L) |
| 02 | Micromoles per litre (µmol/L) |
| 03 | Micrograms per litre (µg/L) |
| 04 | Micrograms per millimole (µg/mmol) |
| 05 | Microgram albumin per hour (µg/ml/hr) |
| 06 | Microgram albumin per minute (µg/min) |
| 07 | Microgram albumin per 24 hours (µg/24hr) |
| 08 | Number (Retired September 2013) |
| 09 | Percentage (%) |
| 10 | Kilograms (kg) |
| 11 | Metres (m) |
| 12 | Picograms (pg) |
| 13 | Square Metres (m2) |
| 14 | Millilitres per Minute (ml/min) |
| 15 | Millimetres of mercury (mmHg) |
| 16 | Litres (l) |
| 17 | Beats per minute (bpm) |
| 18 | Centimetres (cm) |
| 19 | Milligrams (mg) |
| 20 | Millilitres (ml) |
| 21 | Minutes |
| 22 | Celsius (ºC) |
| 23 | Millimetres (mm) |
| 24 | Grams per decilitre (g/dl) |
| 25 | Grams per litre (g/l) |
| 26 | Milligrams per litre (mg/l) |
| 27 | Nanograms per millilitre (ng/ml) |
| 28 | International Units per litre (IU/L) |
| 29 | Decilitres (d/l) |
| 30 | Square Millimetres (mm2) |
| 31 | Millilitres (ml) (Retired September 2013) |
| 32 | Grays (Gy) |
| 33 | International Units per kilogram (IU/kg) |
| 34 | Grams (g) |
| 35 | Kilocalories (kcal) |
| 36 | Millimoles (mmol) |
| 37 | Millimoles per mole (mmol/mol) |
| 38 | Picomoles per litre (pmol/L) |
| 39 | Milligrams per millimole (mg/mmol) |
| 40 | Nanograms per litre (ng/l) |
| 41 | Micrograms per millilitre (µg/ml) |
| 42 | Millimetres of water (mmH2O) |
| 43 | Cubic Millimetres (mm3) |
| 44 | Litres per week per 1.73 metres squared (l/week/1.73²) |
| 45 | Millilitres per Minute divided by 1.73 Square Metres (ml/min/1.73m2) |
| 46 | number times ten raised to the power of nine per litre (x109/l) |
| 47 | 5 Millimetres Squared |
| 48 | Grams per kilogram per day (g/kg/day) |
| 49 | Kilopascals (KPa) |
| 50 | Femtolitres (fl) |
| 51 | Megavolts |
Change to Data Element: Changed Description
| Format/Length: | an1 |
| National Codes: | See DETRUSOR MUSCLE PRESENCE INDICATION CODE |
| Default Codes: | X - Not applicable (Not possible / appropriate to provide any other applicable code) |
| 9 - Not Known (Not recorded or test not done) (Retired 1 April 2020) |
Notes:
DETRUSOR MUSCLE PRESENCE INDICATION CODE is the same as attribute DETRUSOR MUSCLE PRESENCE INDICATION CODE.
Change to Data Element: Changed Description
| Format/Length: | min an1 max an32 |
| National Codes: | |
| Default Codes: |
Notes:
PROFESSIONAL REGISTRATION ENTRY IDENTIFIER (CANCER FIRST SEEN) is the same as attribute PROFESSIONAL REGISTRATION ENTRY IDENTIFIER.
PROFESSIONAL REGISTRATION ENTRY IDENTIFIER (CANCER FIRST SEEN) is the registration identifier allocated by an Organisation for the CARE PROFESSIONAL who first sees the PATIENT following the initial referral which leads to the cancer diagnosis.PROFESSIONAL REGISTRATION ENTRY IDENTIFIER (CANCER FIRST SEEN) is the registration identifier allocated by an Organisation for the CARE PROFESSIONAL who first sees the PATIENT following the initial referral which leads to the cancer diagnosis.
Change to Data Element: Changed Description
| Format/Length: | min an1 max an32 |
| National Codes: | |
| Default Codes: |
Notes:
PROFESSIONAL REGISTRATION ENTRY IDENTIFIER (TREATMENT) is the same as attribute PROFESSIONAL REGISTRATION ENTRY IDENTIFIER.
PROFESSIONAL REGISTRATION ENTRY IDENTIFIER (TREATMENT) is the registration identifier allocated by an Organisation for the CARE PROFESSIONAL responsible for the treatment of the PATIENT.PROFESSIONAL REGISTRATION ENTRY IDENTIFIER (TREATMENT) is the registration identifier allocated by an Organisation for the CARE PROFESSIONAL responsible for the treatment of the PATIENT.
Change to Data Element: Changed Description
| Format/Length: | an2 |
| National Codes: | |
| Default Codes: |
Notes:
PROFESSIONAL REGISTRATION ISSUER CODE (PATHOLOGY REPORT AUTHORISED BY) is the same as attribute PROFESSIONAL REGISTRATION BODY CODE.
PROFESSIONAL REGISTRATION ISSUER CODE (PATHOLOGY REPORT AUTHORISED BY) is the code which identifies the PROFESSIONAL REGISTRATION BODY of the CARE PROFESSIONAL who authorised the pathology report.PROFESSIONAL REGISTRATION ISSUER CODE (PATHOLOGY REPORT AUTHORISED BY) is the code which identifies the PROFESSIONAL REGISTRATION BODY of the CARE PROFESSIONAL who authorised the pathology report.
Permitted National Codes:
| 02 | General Dental Council |
| 03 | General Medical Council |
| 04 | General Optical Council |
| 08 | Health and Care Professions Council |
| 09 | Nursing and Midwifery Council |
Change to Data Element: Changed Description
| Format/Length: | an2 |
| National Codes: | |
| Default Codes: |
Notes:
PROFESSIONAL REGISTRATION ISSUER CODE (PATHOLOGY TEST REQUESTED BY) is the same as attribute PROFESSIONAL REGISTRATION BODY CODE.
PROFESSIONAL REGISTRATION ISSUER CODE (PATHOLOGY TEST REQUESTED BY) is the code which identifies the PROFESSIONAL REGISTRATION BODY of the CARE PROFESSIONAL who requested the pathology test.PROFESSIONAL REGISTRATION ISSUER CODE (PATHOLOGY TEST REQUESTED BY) is the code which identifies the PROFESSIONAL REGISTRATION BODY of the CARE PROFESSIONAL who requested the pathology test.
Permitted National Codes:
| 02 | General Dental Council |
| 03 | General Medical Council |
| 04 | General Optical Council |
| 08 | Health and Care Professions Council |
| 09 | Nursing and Midwifery Council |
Change to Data Element: Changed Description
| Format/Length: | an2 |
| National Codes: | |
| Default Codes: |
Notes:
PROFESSIONAL REGISTRATION ISSUER CODE (TREATMENT) is the same as attribute PROFESSIONAL REGISTRATION BODY CODE.
PROFESSIONAL REGISTRATION ISSUER CODE (TREATMENT) is the code which identifies the PROFESSIONAL REGISTRATION BODY of the CARE PROFESSIONAL responsible for the treatment of the PATIENT.PROFESSIONAL REGISTRATION ISSUER CODE (TREATMENT) is the code which identifies the PROFESSIONAL REGISTRATION BODY of the CARE PROFESSIONAL responsible for the treatment of the PATIENT.
Permitted National Codes:
| 02 | General Dental Council |
| 03 | General Medical Council |
| 04 | General Optical Council |
| 08 | Health and Care Professions Council |
| 09 | Nursing and Midwifery Council |
For enquiries about this Change Request, please email information.standards@nhs.net